Welcome to Matrix Education
To ensure we are showing you the most relevant content, please select your location below.
Select a year to see courses
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Get HSC Trial exam ready in just a week
Get HSC exam ready in just a week
Select a year to see available courses
Science guides to help you get ahead
Science guides to help you get ahead
In this article, we give you the history and uses for the elements in Group 2 – Alkaline Earth Metals.
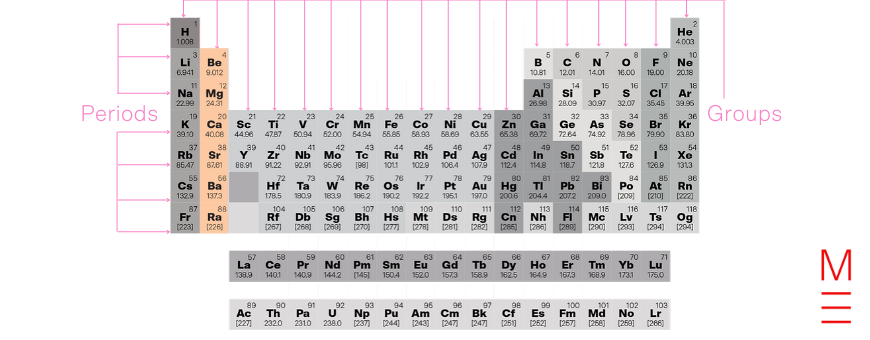
Group 2 contains the alkaline earths. Earths was an old term that meant oxides, and the group’s name comes from noticing that oxides of these metals react with water to form alkaline products (i.e. hydroxides).
Group 2 elements have 2 electrons in their outermost shell. They form cations with a charge of +2, and reactivity increases down the group as the valence electrons become more easily removed due to a weaker attraction to the nucleus.
Group 1 and group 2 together form the s-block due to the valence electrons occupying an s orbital.

All the key Chemistry concepts and formulas in one foldable document!

Fill out your details below to get this resource emailed to you.
"*" indicates required fields
Click on the following elements to learn more about them:
Emeralds and the mineral beryl were known since ancient times and were noted for their similarities. In the 1700s, both were mistaken as aluminium silicate compounds, however in 1798, Louis-Nicolas Vauquelin discovered that they both contained a new element. He named this glucinium after the Greek glyko- meaning sweet, as some beryllium compounds tasted sweet, and the symbols G and Gl were used. Another scientist who had investigated beryl and emeralds, Martin Heinrich Klaproth, proposed the name beryllina after the mineral from which it derived. But, it was ultimately Friedrich Wöhler, one of the first to isolate the element in 1828, who gave beryllium its current name. Beryl in turn derives its name from the Indo-European word for pale, which describes its appearance.
Beryllium is used in alloys, such as lightweight alloys in the aerospace industry or copper alloys to increase strength and reduce sparking. It is transparent to X-rays and is used in X-ray tubes and detectors. It is also used in nuclear applications as a neutron source or reflector.

Joseph Black first distinguished magnesium as an element by noting the difference between magnesium oxide (magnesia) and calcium oxide in 1755. Magnesium metal was isolated in 1808, by Sir Humphry Davy. The name derives from the region of Magnesia in Greece, which was the source of several minerals: magnesia negra (black magnesia, which is manganese dioxide), magnesia alba (white magnesia, which is magnesium oxide), and magnetite (which is a magnetic iron oxide).
Magnesium is used in light-weight metal alloys, notably for the aerospace and automotive industries, and for consumer electronics. It is also used as a firestarter and in pyrotechnics due to its flammability. Magnesium has a biological role and is an important nutrient, and a component of chlorophyll.

Calcium compounds such as lime (calcium oxide and hydroxide), gypsum (calcium sulfate), and chalk, limestone and marble (calcium carbonate) have been known and used since ancient times. In 1787, Antoine Lavoisier proposed that chaux, calcium oxide, was the oxide of a new element, which was subsequently isolated in 1808 by Sir Humphry Davy who formed a calcium-mercury amalgam and then evaporated the mercury to leave behind the calcium. He named it after the Latin calcis or calx, meaning limestone.
The main industrial use of metallic calcium is in steel making and the production of other metals such as chromium and zirconium. Calcium minerals form teeth and bones, and limestone and marble were used widely in construction.

In 1790, Adair Crawford and William Cruickshank noticed that ore used to produce barium from the Scottish village of Strontian exhibited different properties compared to ores derived from other places. Crawford proposed that it contained a new element that was yet to be discovered. The same conclusion was arrived at by Friedrich Gabriel Sulzer and Johann Friedrich Blumenbach, who named the mineral strontianite. In 1793, Thomas Charles Hope proposed the new element in strontianite be named strontites. Strontium was first isolated in 1808 by Sir Humphry Davy, who changed the suffix to -ium to be consistent with the names of other alkaline earths.
The very few uses of strontium include glow-in-the-dark materials, fireworks and toothpaste for sensitive teeth. It was previously used on a large scale in sugar manufacturing and in CRT television screens, but in both cases the technology is now obsolete.

Barium minerals were known since at least the middle ages. Barium hydroxide was known as baryta from the Greek barys meaning heavy. In 1774, Carl Scheele concluded that another mineral, baryte (barium sulfate) contained a new element but only isolated barium oxide. Sir Humphry Davy succeeded in isolating barium metal in 1808 and named it by modifying the name baryta with the suffix -ium used for metals.
Barium was commonly used in vacuum tubes to react with gases that entered, but this technology is mostly obsolete. Barium sulfate is used as a drilling fluid in oil drilling, and as a radiocontrast agent for X-ray imaging of the digestive system. Barium is also used in superconductors and fireworks.

Marie and Pierre Curie discovered radium in 1898 in the uranium mineral pitchblende. They found the material was still radioactive after removing the uranium from it, and continued to remove other components until a radioactive material similar to barium remained, which they identified as a new element. They named it radium as a reference to its ability to emit radiation. In 1910, Marie Curie and Andre-Louis Debierne isolated radium as a metal.
Radium was initially considered a health supplement because it released energy and was added to some food, drinks and household products. It was also used in luminous paint, most famously in watch dials. Once it was recognised as a serious health hazard, its use was phased out. Radium is now only used in very specific applications including radiotherapy and as a radiation source. The most stable isotope has a half-life of 1600 years.

Matrix+ will give you access to HSC Chemistry Experts, wherever you are! Learn more.
Start HSC Chemistry confidently
Expert teachers, comprehensive resources, one-to-one help! Learn from home with Matrix+ Online.
© Matrix Education and www.matrix.edu.au, 2025. Unauthorised use and/or duplication of this material without express and written permission from this site’s author and/or owner is strictly prohibited. Excerpts and links may be used, provided that full and clear credit is given to Matrix Education and www.matrix.edu.au with appropriate and specific direction to the original content.