Year 12 Chemistry
Boost your Chemistry marks and confidence with structured courses online or on-campus.
Learning methods available
Select a year to see available courses
In this article, we give you the history and uses for the elements in Groups 13-16.
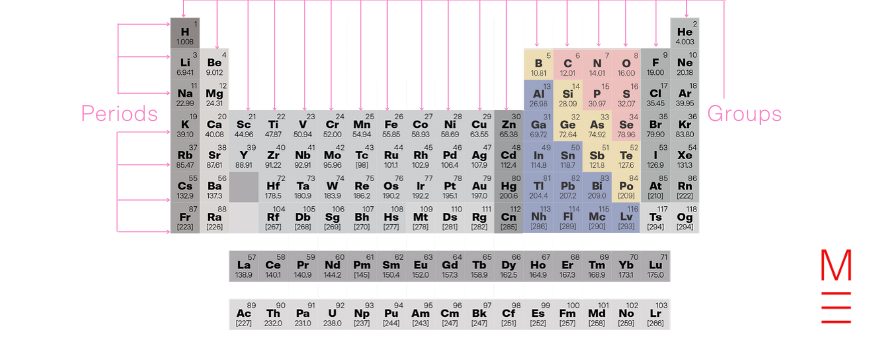
Groups 13-16 don’t really fit together in any nice way, so let’s look at them one by one. The thing they have in common is the progressive filling of a p-orbital. The metallic properties of these elements decrease along each period and increase down each group. As a result, these groups contain non-metals like nitrogen, oxygen and sulfur, all the semimetals, and metals like aluminium, tin, lead, and bismuth.
Together with groups 17 and 18, these elements form the p-block.
Click on the following elements to learn more about them:
| Group → Period ↓ |
13 | 14 | 15 | 16 |
| 2 | Boron | Carbon | Nitrogen | Oxygen |
| 3 | Aluminium | Silicon | Phosphorus | Sulfur |
| 4 | Gallium | Germanium | Arsenic | Selenium |
| 5 | Indium | Tin | Antimony | Tellurium |
| 6 | Thallium | Lead | Bismuth | Polonium |
| 7 | Nihonium | Flerovium | Moscovium | Livermorium |
Although boron-containing minerals were known historically, boron compounds were not used widely prior to the 19th century when the mineral borax became widely available. In 1808 boron was isolated independently by Joseph Louis Gay-Loussac and Louis Jacques Thenard, and Humphry Davy who proposed the name boracium, after the mineral borax. The name boron was adopted by analogy with carbon.
Boron is used as an additive in glass, particularly fiberglass and borosilicate glass, and in ceramics. Boron compounds such as borax and boric acid are used domestically as cleaning products, insecticides, and food additives amongst other uses.
Carbon was known since prehistoric times in the form of charcoal, however it was not known that diamonds and graphite are the same element. In 1772, Antoine Lavoisier showed that diamonds and charcoal produced the same reaction products when burnt. Similarly, in 1786, Claude Louis Berthollet, Gaspard Monge, and C. A. Vandermonde did the same with graphite, previously believed to be a type of lead. They proposed the name carbone after the Latin carbo meaning charcoal.
Carbon’s main industrial uses are as a fuel (coal, oil, and gas) and for making plastics, it is combined with iron to make steel, and diamonds are used in cutting and drilling tools. Carbon fibres are used in woven materials. Carbon has a crucial biological role and all plant and animal products contain carbon.
Carbon fibre, anthracite coal, charcoal, graphite brushes from a motor and graphite electrodes, low density polyethylene and different allotropes of carbon: diamonds (loose), diamond-tipped drill bit, graphite, fullerenes (carbon nanotubes), graphene (in solution)
Ammonia, nitric acid and nitrate salts were known, in some cases, since ancient times. Even though 78% of the Earth’s atmosphere is nitrogen gas, it was only discovered in 1772 by Daniel Rutherford who called it noxious air. Rutherford did not identify it as an element and several other contemporary scientists also investigated it and proposed the names burnt air and phlogisticated air. Antoine Lavoisier proposed the name azote from Greek meaning no life, as animals and flames died in pure nitrogen, and whilst this was adopted in other languages, only the chemical term azo- was adopted in English, as did the name pnictogen (Greek for producing suffocation) for the group to which nitrogen belongs. In 1790, Jean-Antoine Chaptal proposed the name nitrogene from nitre, the French name for potassium nitrate, and Greek -gene meaning to produce. The association was that nitre was used to make an acid (nitric acid) of which nitrogen was a key element.
Nitrogen compounds have a huge range of applications from fertilisers to explosives. Elemental nitrogen is used as an inert atmosphere and as a cryogenic liquid.
Various scientists investigated the nature of air and oxygen in the 17th and 18th centuries. Michael Sendivogius may have isolated oxygen gas and recognised that it is essential for life, although his discovery was rejected by other scientists. Carl Wilhelm Scheele produced oxygen in 1771 and named it fire air, but did not publish the result until 1777. In 1774, Joseph Priestly produced oxygen in a similar way to Scheele, named it dephlogisticated air, and associated it with increased combustion. Priestly published his results in 1775, before Scheele. Antoine Lavoisier also claimed to have independently discovered oxygen around 1774 and proposed it to be an element.
Lavoisier proposed that air is a mixture of two gases – vital air required for combustion and breathing, and azote (nitrogen) which did not support combustion or breathing. He named vital air oxygene after Greek oxy meaning acid, and gene meaning to produce. This was due to a mistaken belief that all acids contained oxygen. It was later shown that acids contain hydrogen, not oxygen, but the name was already established and was not changed.
Oxygen is essential for life and found in a large number of compounds. Oxygen gas itself is used for life support in medical settings. In industrial settings, it is used in the production of steel, the production of various organic compounds, and for welding and rocket fuel.
Aluminium is the most common metal in the Earth’s crust and found in over 270 minerals, though not in pure form owing to its high reactivity. The mineral alum (containing aluminum and sulfate) was used since 5th century BC Greece in the dyeing of fabrics and continued to be of commercial importance into the 16th century. Various scientists proposed that alum was the salt of a new element, and Hans Christian Ørsted first isolated the metal in 1824. Aluminum remained scarce and more expensive than gold until modern methods of extracting it from the minerals bauxite and alumina (aluminium hydroxide and oxide) were developed in the late 1800s.
The name ultimately derives from the Proto-Indo-European alu- meaning bitter. Sir Humphry Davy proposed the name alumium in 1808, but changed it to aluminum in 1812 to acknowledge the mineral alumina. In the same year Thomas Young proposed changing the name to aluminium, which became the standard name in 1990, though aluminum remains an accepted variant and is used in American and Canadian English.
Aluminium is the second most commonly used metal (after iron) owing to its ability to form strong, lightweight, corrosion-resistant alloys. It is used in the construction of buildings, vehicles, food packaging, household items, power lines, and other equipment and machinery.
Although silicon is the second most abundant element in the Earth’s crust and its compounds had been used for thousands of years, it was not recognised as an element.
In 1787, Antoine Lavoisier suggested that silica (SiO2) was an oxide of a new element. Humphry Davy attempted to isolate it in 1808 and gave it the name silicium, combining the Latin silex meaning flint, and the suffix –ium to indicate a metal. It is likely that Joseph Louis Gay-Lussac and Louis Jacques Thénard produced elemental silicon in 1811, but failed to identify it as an element.
In 1817, the name of the yet-to-be-discovered element was changed to silicon by Thomas Thomson, as he believed it to be a non-metal like boron or carbon. Finally, in 1823 Jöns Jacob Berzelius used a very similar method to Gay-Lussac to produce amorphous silicon and is credited with its discovery.
Silicon-containing minerals are found everywhere on Earth in the form of clay, sand, and various kinds of stone. These are widely used in construction (bricks, stone, cement), ceramics, porcelain, and glass. Silicones are also used in applications ranging from industrial uses like lubricants to implants. High purity silicon is used in the electronics industry for making integrated circuits and their components, which underpin all electronic devices, and for making solar panels. High purity silica glass is used for optical fibres for telecommunications.
Phosphorus was discovered in 1669 by Hennig Brand, an alchemist attempting to create gold from the salts he extracted from his own urine. He instead produced a white material which was the white allotrope of phosphorus. He observed that it glowed in the dark and named it after the Greek phosphorus meaning light-bearer. Antoine Lavoisier recognized that phosphorus is an element in 1777. Note that despite the name, the glow observed by Brand was chemiluminescence (light released during the reaction of white phosphorus and air), not phosphorescence.
Phosphorus compounds are used predominantly as fertilisers, but also as food additives such as baking powder, detergents and pesticides. Elemental red phosphorus replaced white phosphorus in most uses (the most familiar being matches) as it is more stable and less toxic.
Sulfur may be found in pure form on Earth and was known since ancient times in India, Greece, Egypt and China. In 3rd century China, sulfur was extracted from the mineral pyrite (FeS2), and in the 11th century it was used by the Chinese in gunpowder. In 1777, Antoine Lavoisier proposed that sulfur was an element. Sulfur is associated with burning, as it is flammable and perhaps through association with volcanoes. The earlier English name brimstone (burning stone) was replaced by the French sulfere which derives from earlier Latin names that likely refer to burning. The alternate spelling, sulphur, is used in British English and incorrectly suggests a Greek origin for the name. The chemical prefix thio- is in fact what derives from the Greek name theio.
Sulfur is mainly used to produce sulfuric acid which has many important industrial applications, primarily the production of fertilisers. Other applications include insecticides and fungicides, the production of polymers, vulcanization of rubber, food production and detergents.
Gallium was predicted to exist by Dmitri Mendeleev in 1871, who gave it the name eka-aluminium and correctly predicted many of its properties. In 1875, it was discovered by French chemist Paul Emile Lecoq de Boisbaudran using spectroscopy of a mineral, before isolating a pure sample. Boisbaudran named the element gallia after the Latin Gallia meaning Gaul (France).
The main application for gallium is in the production of semiconductors for electronics and for infrared optics.

Dmitri Mendeleev predicted the existence of germanium and named it ekasilicon (Es) in 1869. In 1885, the mineral argyrodite (Ag8GeS6) was discovered in Germany and named as such as it contained silver, however Clemens Winkler identified that it also contained sulfur and a new element. He isolated this element, but it was unclear which of the predicted elements this was and where it belonged in the periodic table. Theodor Hieronymus Richter and Julius Lothar Meyer identified that its properties matched Mendeleev’s predictions for ekasilicon very closely. Winkler wanted to name the element neptunium, but that name had already been used for prior element discovery claims that had turned out to be false. He instead chose the name germanium, after Germany, his homeland, however that led to some confusion about whether the name was geranium, after the flower.
The main use of germanium is in fibre optics used for communications, and other optics applications and solar cells. Although the electronics industry was initially based on germanium transistors, it has been replaced by silicon.
Arsenic was known and used since ancient times as it was alloyed with copper to make bronze, and various arsenic compounds were used as pigments and as poisons. It is unclear who identified it as an element – Zosimos of Panopolis described the production of arsenic in 300 AD, as did Jabir ibn Hayyan in 815 AD. Its name is ultimately derived from the Persian zarnik, and subsequent Arabic al-zarnik, which described the gold-coloured appearance of orpiment (As2S3) pigment.
Arsenic continued to be used in cosmetics and in pigments but was phased out after its toxicity was better understood. It was then used for insecticides and subsequently phased out in most cases. Its main current uses are as a dietary supplement for livestock and in GaAs semiconductors.
Around 1817, Jons Jacob Berzelius and Johan Gottlieb Gahn noticed a red precipitate was being produced in their chemical plant where they produced sulfuric acid. They assumed this was an arsenic compound, however, when it was burnt, the smell was similar to burning tellurium compounds. This led Berzelius to conclude that it was tellurium, however the mines from which they were obtaining their reactants were not known to contain tellurium minerals. Berzelius investigated the material further and concluded it was a new element with properties similar to tellurium and sulfur. He named it selenium after the Greek selene, meaning the Moon, in analogy with tellurium which is named after the Earth.
The main use of selenium is in glass-making, as it gives glass a red colour. It is also used in shampoo and in dietary supplements for humans and livestock (although it is toxic in higher doses). It was previously used in light meters and in electronics, but it was eventually replaced with other materials.
Ferdinand Reich and Hieronymous Theodor Richter discovered indium when testing various ores for their possible thallium content in 1863. Reich intended to use spectroscopic techniques to look for the green emission lines characteristic of thallium. Richter undertook the observations as Reich was colourblind, and observed a bright blue line instead. The line did not match any elements known at the time, leading to the conclusion that it was from a new element. They named the element indium from indigo, reflecting the colour of the emission line, with the -ium suffix. The name derives from Greek indikos meaning Indian, a reference to the Indian origin of indigo dye. Richter first isolated the element the following year, and later disputed Reich’s claim as co-discoverer.
The main use of indium is as indium oxide or indium tin oxide transparent conducting films used in electronics for flat panel displays such as touch screens.

Tin has been known since the Bronze Age, from around 3500 BC, used in the production of bronze, and was isolated as a metal since around 600 BC. Arsenic was initially alloyed with copper to make bronze, but was replaced by tin as tin is non-toxic. The name tin derives from the Germanic word tinom, of unknown origin. In Latin tin was called stannum, which was originally the name of a lead-silver alloy. The element symbol Sn and the names stannous or stannic given to tin compounds derive from this.
Tin is used in low-melting point alloys such as solder, and for plating steel to prevent corrosion, such as in tin cans.
Antimony was known since ancient times through the compound antimony sulfide (Sb2S3) which was used in Ancient Egyptian eye cosmetics (known as kohl), and also in its metallic form. The origin of the name is unclear and different names appear to have been used over time. The Greek words antimonachos (meaning anti-monk, as alchemists tended to be monks and may have been poisoned by antimony) or antimonos (anti-alone, as it was used in alloys) are believed to have resulted in the name antimonium. The Egyptian word stim, which led to the Greek stimmi, and stibi may have given rise to the name stibium, of which the symbol Sb is an abbreviation.
Antimony is mainly used in flame retardants, and also in alloys, most commonly with lead, such as in lead-acid battery plates, and in ammunition.
In 1782, Franz-Joseph Muller von Reichenstein, who was chief inspector of mines in Transylvania, investigated a mineral from a gold mine in that region called aurum paradoxum (paradoxical gold) or metallum problematum (problematic metal). The ore was believed to contain antimony but did not display the expected properties (hence the problematum). He eventually concluded that it must contain a new element similar to antimony. In 1784, Torbern Bergman confirmed that the mineral did not contain antimony, but he passed away before his work progressed further. In 1798, von Reichenstein sent a sample of the mineral to Martin Heinrich Klaproth who succeeded in isolating the new element and named it tellurium after Tellus, a Latin name for the mythological mother Earth. The original mineral von Reichenstein was investigating was gold telluride (AuTe2). Prior to Klaproth’s work, in 1789, Pal Kitaibel independently discovered the element in a mineral believed to contain molybdenum, but acknowledged von Reichenstein’s discovery.
The main use of tellurium is in steel, copper and lead alloys to improve machinability and corrosion resistance. It is also used to make semiconductors for applications such as solar panels and in optics for infrared sensors and optical storage (e.g. rewritable DVDs).
Thallium was discovered independently by William Crookes and Claude Auguste Lamy. They both used spectroscopy to identify the presence of the new element, and they were both studying residues formed during the production of sulfuring acid. This resulted in some tension between the two scientists. Crookes was the first to publish the results in 1861, but Lamy was the first to isolate it as a metal. Lamy received an award for his discovery, causing Crookes to protest and also receive an award. It was Crookes who named the element thallium, after the Greek thallos meaning a green shoot or twig, as a green spectral line had been used to identify the element.
Thallium compounds are highly toxic and were initially used as pesticides and insecticides, however they were phased out since the 1970s due to their toxicity to humans. Very few niche applications remain such as in electronics, medical tests, and low temperature thermometers.
The earliest use of lead dates back to the 7th millennium BC in Asia Minor, and it was known and used by all early civilisations. The Ancient Egyptians used lead in cosmetics by grinding the mineral galena (PbS2), in fishing, pottery and glass making. It was also used as currency and in construction, particularly for plumbing, for slingshot bullets and for writing. Over time, lead came to be confused with other materials such as bismuth, tin (referred to as bright lead), graphite (black lead) or antimony. The name lead derives from the Germanic lauda meaning a weight, whereas the symbol Pb comes from the Latin plumbum, which referred to the metal itself, pipes made of the metal, and pencils made of graphite which was believed to be a type of lead.
More recently lead has been used for ammunition, ballasts, construction, radiation shielding, lead glass and lead-acid batteries, however it has been phased out of many applications due to its toxicity.
Bismuth has been known since ancient times but was sometimes confused with lead and tin owing to similarities between them. The origin of the name bismuth is unclear and either derives from the Arabic bi ismid, meaning similar to antimony or from the German weisse masse or Wismuth, meaning white mass. It was thought to be the heaviest element with stable isotopes, until 2003 when it was discovered that it is radioactive with a half life of 2 x 1019 years, which is over a billion times longer than the age of the universe.
Bismuth is used in pharmaceuticals, in cosmetics and pigments, and as a replacement for lead in ammunition.
Polonium was discovered in 1898 by Pierre and Marie Curie when studying the uranium mineral pitchblende. They removed the uranium and thorium from pitchblende and found that the remaining material was still radioactive, and in fact had a higher activity per gram than before the other elements were extracted. They attributed this activity to a new element which they named polonium after Marie’s native homeland, Poland (Polonia in Latin). Their aim was to bring attention to Poland’s struggle for independence as at the time it was under Russian, German, and Austro-Hungarian control. Several years later, polonium was independently discovered by Willy Marckwald, and was named radiotellurium, however it was eventually realised that he had in fact just rediscovered polonium. Polonium is highly radioactive and only exists naturally in trace quantities as a decay product of other unstable elements. The Po-210 isotope has the longest half-life of the naturally occurring isotopes, of approximately 140 days.
Polonium has been used as a radiation source, and due to its high radioactivity, in thermoelectric generators.
Around 2002 a collaboration between the Joint Institute for Nuclear Research in Dubna and the Lawrence Livermore Laboratory in Livermore, California bombarded americium with calcium and claimed to have produced element 115, which then alpha-decayed to element 113. In 2003 the Riken lab in Japan bombarded bismuth with zinc and detected one atom of element 113. After a long period of review and further experiments by Dubna, Livermore, Riken, and other laboratories, IUPAC recognised Riken for the discovery in 2015.
Riken considered the names japonium, after Japan, nishinanium, after physicist Yoshio Nishina, and rikenium, after the Riken institute. In 2016, japonium was the preferred name, but was dropped as the abbreviation jap was considered derogatory. The name nihonium was then proposed, which means Japan in Japanese, with the symbol Nh, and was accepted by IUPAC in the same year. The name also recognised Masataka Ogawa who discovered rhenium and had proposed the name nipponium (Np), also meaning Japan. Ogawa’s discovery was overlooked at the time as he erroneously claimed he had discovered technetium (the name nipponium could not be reused, and Np had already been adopted for neptunium).
Nihonium has no applications as it is extremely expensive and difficult to produce and is highly unstable.
Element 114 was first synthesised at the Joint Institute of Nuclear Research in Dubna in 1998 by bombarding plutonium with calcium, in collaboration with the Lawrence Livermore National Laboratory. The discovery was recognised by IUPAC in 2011. The Dubna team proposed the name flerovium after the Flerov Laboratory of Nuclear Reactions (one of the Dubna team’s laboratories), named after its founder physicist Georgy Flyorov for his contributions to heavy ion physics and his co-discovery of spontaneous fission. The name was accepted in 2012.
Flerovium has no applications as it is extremely expensive and difficult to produce and is highly unstable.
The synthesis of element 115 was first claimed by the Joint Institute of Nuclear Research in Dubna in 2003 by bombarding americium with calcium, in collaboration with the Lawrence Livermore National Laboratory, and followed up with a series of experiments ending in 2010. In 2011,i IUPAC rejected the claim of discovery as it considered the results to be insufficient, but recognised Dubna and Livermore for their discovery based on the 2009 and 2010 experiments in 2015 after other laboratories replicated the results.
The names langevinium, honouring physicist Paul Langevin, and moscovium, after the Moscow Oblast where Dubna is located, were proposed. IUPAC accepted the name moscovium in 2016.
Moscovium has no applications as it is extremely expensive and difficult to produce and is highly unstable.
The synthesis of element 116 was first claimed by the Joint Institute of Nuclear Research in Dubna in 2000 by bombarding curium with calcium, in collaboration with the Lawrence Livermore National Laboratory. One atom was detected alpha-decaying to element 114, indicating element 116 as the parent. Similar experiments continued at Dubna until 2006, and at other laboratories until 2009. In 2011, IUPAC recognised Dubna and Livermore for the discovery based on the 2004-2006 results. The name livermorium was adopted in 2012 after the Lawrence Livermore National Laboratory, in turn named after the city of Livermore, California, in turn named after rancher Lawrence Livermore.
Livermorium has no applications as it is extremely expensive and difficult to produce and is highly unstable.
At Matrix, our Year 12 Chemistry course provides you with quality learning and comprehensive resources to help boost your marks and confidence! Learn more now.
Start HSC Chem confidently
Expert teachers, comprehensive resources, one-to-one help! Learn from home with Matrix+ Online.
© Matrix Education and www.matrix.edu.au, 2023. Unauthorised use and/or duplication of this material without express and written permission from this site’s author and/or owner is strictly prohibited. Excerpts and links may be used, provided that full and clear credit is given to Matrix Education and www.matrix.edu.au with appropriate and specific direction to the original content.