Welcome to Matrix Education
To ensure we are showing you the most relevant content, please select your location below.
Select a year to see courses
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Get HSC Trial exam ready in just a week
Get HSC exam ready in just a week
Select a year to see available courses
Science guides to help you get ahead
Science guides to help you get ahead
In this article, we give you the history and uses for the elements in Group 1 – Hydrogen and Alkali Metals.
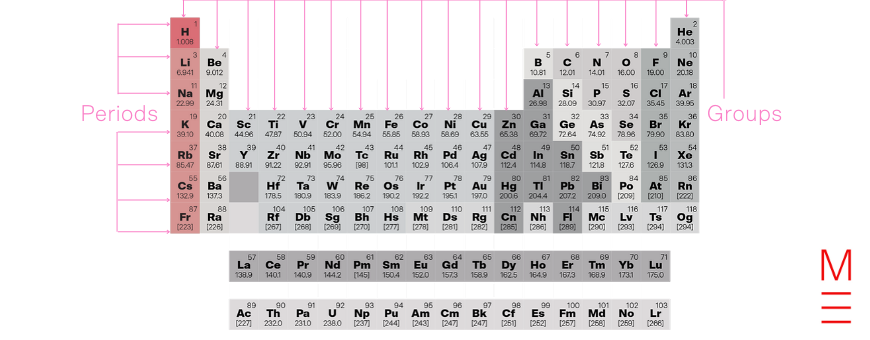
Group 1 contains hydrogen and the alkali metals. The alkali metals are named as such because they form alkaline products (i.e. hydroxides) when they react with water.
Elements in Group 1 have 1 electron in their outermost shell. They form cations with a charge of +1, and reactivity increases down the group as the valence electron becomes more easily removed due to a weaker attraction to the nucleus.
Group 1 and group 2 together form the s-block due to the valence electrons occupying an s orbital.

All the key Chemistry concepts and formulas in one foldable document!

Fill out your details below to get this resource emailed to you.
"*" indicates required fields
Click on the following elements to learn more about them:
Hydrogen is released in reactions between acids and metals, and was identified as a distinct substance in these reactions by Henry Cavendish in 1766. Cavendish named the gas inflammable air. In 1781, he found that water was produced when hydrogen was burned in oxygen. Two years later Antoine Lavoisier named the element hydrogene after the Greek hydro- meaning water, and -genes meaning to create or produce.
Whilst hydrogen is commonly found in water and hydrocarbons, its main use as an element is in processing fossil fuels and fats and oils, and in the production of ammonia for food production.
The lithium-containing mineral petalite was discovered in 1800 by Jose Bonifacio de Andrada e Silva. In 1817, Johan August Arfwedson identified that it contained a new element as it produced similar alkaline compounds to sodium and potassium, but which were less soluble. The alkaline compounds were named lithina by Jons Jakob Berzelius, in whose lab Arfwedson worked, from the Greek lithos meaning stone, reflecting their discovery from a solid mineral. The metal in these compounds was subsequently named lithium, and was first isolated by William Thomas Brande in 1821.
There are many uses for lithium, including in glass and ceramics, lubricants, batteries, as a rocket propellant, and in the production of nuclear energy and weapons. Lithium salts are also used as psychiatric medication.

Sodium compounds such as salt (NaCl), soda (Na2CO3) and lye (NaOH) have been known for millennia, and used domestically in food, medicines, soap making and various other applications. However, as it is highly reactive, sodium metal is not found naturally.
It was first isolated in 1807 through the electrolysis of NaOH by Sir Humphry Davy. Davy proposed the name sodium, after the word soda, which is believed to derive from the Arabic word suda meaning headache, to which soda (i.e. Na2CO3) was a traditional remedy.
The chemical symbol Na derives from the alternative name, natrium or natronium, proposed by Ludwig Wilhelm Gilbert, which is used in other languages. Natrium derives from the Ancient Egyptian word natron, a mixture of sodium salts mined in Wadi El Natron in Egypt and used in the mummification process.
Sodium compounds are primarily used for de-icing (e.g. of roads) and in food (e.g. as salt or in baking). Sodium is also used in medicines and in the production of various organic compounds. Sodium vapour lamps are used in (yellow-coloured) street lighting. Sodium metal itself has few direct uses owing to its high reactivity, with its use as a coolant in some nuclear reactors being one example.

Potash, i.e. the ash from burning plants and wood, was a well-known source of potassium salts. In 1797, Martin Klaproth discovered potash in the minerals leucite and lepidolite and concluded that it was not produced by plants themselves but must be a material associated with an undiscovered element. He named this element kali, after the Arabic word al-kali meaning plant ashes.
In 1807, Sir Humphry Davy isolated the element and named it potassium, as it was obtained from potash. The name kalium was also proposed by others, with the symbol K, and was adopted in some languages, whilst English followed Davy’s name. The Arabic word al-kali (alkali) came to refer to the alkaline substances in general and the group of alkali metals and alkali earths.
Potassium is mainly used in salts utilised as fertilisers in agriculture.

Robert Bunsen and Gustav Kirchhoff discovered rubidium in 1861. They were using flame spectroscopy to study the mineral lepidolite and identified unique spectral lines which they attributed to a new element. They named the element after the Latin rubidus, meaning deep red, to reflect the bright red lines in its emission spectrum.
The most common use of rubidium is in fireworks and in atomic clocks. Rubidium vapour is also used in very specific physics applications such as laser cooling and Bose-Einstein condensates.

Robert Bunsen and Gustav Kirchhoff discovered caesium in a sample of mineral water using spectroscopy in 1860. They observed bright blue lines in its emission spectrum and concluded there was the presence of a new element. They named it caesium after the Latin caesius meaning sky-blue. They went on to obtain 7.3 g of caesium chloride from 44,000 L of mineral water but were unable to isolate the metal. In 1882, one of Bunsen’s students Carl Stterberg succeeded in isolating it.
Currently, the main use of caesium is in fluids used in oil drilling. Caesium is also used in high precision atomic clocks. It was previously used in vacuum tubes to react with any air that entered the tube and hence maintain a vacuum, but vacuum tube technology is mostly obsolete.

The existence of francium was predicted in the late 1800s and the tentative name eka-caesium was used. Francium is highly radioactive and only occurs naturally in extremely small quantities, which led to several false claims of discovery. Radioactive potassium-40 was mistaken for element 87 and named russium in 1925. In 1926, a claim based on X-ray spectroscopy named the element alkalinium, and in 1930, another claim of its discovery in pollucite named it virginium, but its validity was discredited. In 1936, another claim based on X-ray spectroscopy was made, and the name moldavium was put forth. However, this too was discredited and it was suggested that mercury or bismuth were being mistaken for the new element.
Element 87 was finally discovered by Marguerite Perey in 1939 who observed unexpected radiation coming from a sample of actinium. She eliminated all known radioactive elements as a source and concluded that the unidentified radiation was coming from element 87, which she named actinium-K. Subsequently, she proposed the name catium (Cm) as a reference to cations, but this was criticised as it sounded like a reference to cats. Perey then named the element francium as she was French. The original symbol Fa was changed to Fr.
Francium is extremely rare and highly unstable; the most stable isotope has a half-life of 22 minutes. It has no known uses.
At Matrix, our specialised HSC Chemistry teachers, comprehensive resources and engaging lessons will help boost your marks and confidence! Learn more.
Start HSC Chemistry confidently
Expert teachers, comprehensive resources, one-to-one help! Learn from home with Matrix+ Online.
© Matrix Education and www.matrix.edu.au, 2025. Unauthorised use and/or duplication of this material without express and written permission from this site’s author and/or owner is strictly prohibited. Excerpts and links may be used, provided that full and clear credit is given to Matrix Education and www.matrix.edu.au with appropriate and specific direction to the original content.