Welcome to Matrix Education
To ensure we are showing you the most relevant content, please select your location below.
Select a year to see courses
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Get HSC Trial exam ready in just a week
Get HSC exam ready in just a week
Select a year to see available courses
Science guides to help you get ahead
Science guides to help you get ahead
In this article, we give you the history and uses for the elements in Group 17 – Halides.
Group 17 is the halogens, they have 7 electrons in their outermost shell. They are all non-metals, and form anions with a -1 charge. Reactivity decreases down the group, with fluorine being the most reactive.
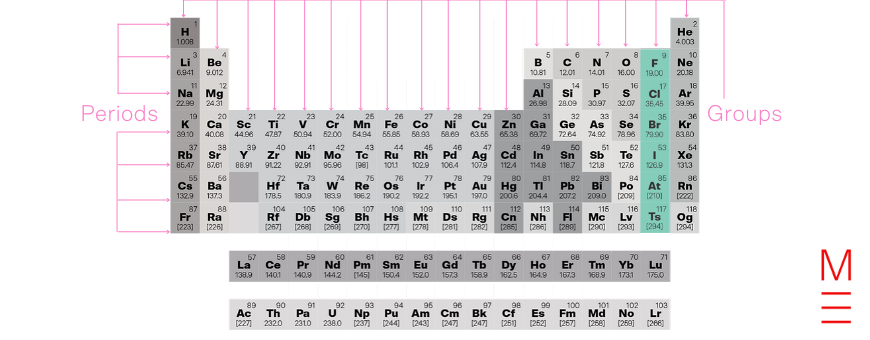
Together with groups 13-16 and 18, they form the p-block.

All the key Chemistry concepts and formulas in one foldable document!

Fill out your details below to get this resource emailed to you.
"*" indicates required fields
Click on the following elements to learn more about them:
Fluorite (CaF2) was used in the 16th century in the processing of metals as an additive to lower their melting points and make them easier to process. It was named fluores after the Latin word fluor, meaning flow. In the 18th century hydrofluoric acid (then called fluorspar acid), made from fluorite, was used in glass etching. Andre Marie Ampere proposed in 1810 that this acid consisted of hydrogen and another element similar to chlorine, and Sir Humphry Davy named this new element fluorine, combining fluo- from the name of the acid and the -ine suffix used in naming halogens. Several scientists then attempted to isolate fluorine from hydrofluoric acid, but were either injured or killed by it, as it is a very toxic substance. Although experiencing serious poisoning, Henri Moissan was the first to successfully isolate fluorine gas in 1886.
Fluorine compounds are still used in the processing of metals. The fluorine-containing polymer PTFE (Teflon) is used in electrical insulation, medical devices and cookware. Fluorine salts are used in tap water and toothpaste to prevent tooth decay. Fluorine is also used in uranium enrichment and in refrigerants (CFCs) though the latter have been phased out as they damage the ozone layer.
Chlorine compounds like salt have been used since ancient times, and chlorine gas was produced from the 1200s as a by-product of acid production, however its discovery as an element began in 1774 when Carl Wilhelm Scheele produced chlorine gas and called it dephlogisticated muriatic acid air. Scheele failed to recognise it as an element and it was eventually believed to be an oxide of the hypothetical element muriaticum. In 1809, Joseph Gay-Lussac and Louis-Jacques Thenard tried to release muriaticum from muriatic acid air and failed, raising the possibility that it was itself an element. The following year Sir Humphry Davy concluded that it is an element, which he named chlorine from the Greek chloros meaning pale green-yellow, which is the colour of chlorine gas. The alternative name halogen was proposed, meaning salt-producer, but instead came to refer to the group to which chlorine belongs.
Chlorine compounds have a very large number of applications in industry in the production of organic compounds, plastics like polyvinyl chloride (PVC), for precursors used in the purification of metals, and as bleaches and disinfectants, such as in the sanitation of drinking water.
Two scientists are credited with independently discovering bromine, Carl Jacob Lowig in 1825 and Antoine Balard in 1826. Lowig extracted bromine from mineral water and submitted his discovery as part of a job application. The publication of his results was delayed, leading to Balard’s results being published first. Balard extracted bromine from seaweed that was used to produce iodine, and found that its properties were in between those of chlorine and iodine. He initially tried to prove it was iodine chloride but after failing to do so, he concluded that he had discovered a new element. He originally named the element muride, after the Latin word for brine, but later changed it to brome after the Greek bromos meaning stench, to describe the smell of bromine vapour.
The main application of bromine is in flame retardants. It is also used in photographic film in the form of silver bromide.
Iodine was first isolated by French chemist Bernard Courtois in 1811 from seaweed, in the process of extracting saltpeter for use in gunpowder for the Napoleonic Wars. Courtois suspected this was a new element and sent samples to other scientists to investigate. In 1813 Joseph Louis Gay-Lussac announced that iodine was either a new element or an oxygen compound. This was followed by an announcement from Humphry Davy claiming it was a new element. The element was named by Gay-Lussac, from Greek iode meaning violet, which is the colour of iodine vapour.
Iodine is used as a disinfectant and a radiocontrast material in medicine, and as a nutritional supplement. Silver iodide is used in photographic film and in cloud seeding to modify rainfall.
The existence of astatine was indirectly predicted by Dmitri Mendeleev, and prior to its discovery it was referred to as eka-iodine. Astatine is extremely unstable and rare, which led to several false discoveries. A 1931 false discovery at the Alabama Polytechnic Institute named the element alabamine, Ab, but was disproven in 1934. In 1937, another such false discovery in Dhaka gave the name dakin, but was also disproven. In 1936, another claim suggested the name dor, or dorine but was eventually rejected as although their sample would have contained the element, their means of detection should have failed. Two further claims, helvetium in 1940 and anglohelvetium in 1942, were also rejected as other scientists were unable to reproduce the results.
In 1940, Dale Corson, Kenneth Ross MacKenzie and Emilio Segre at the University of California, Berkeley, were able to artificially produce the element using nuclear reactions. This came to be recognised as the discovery. The three scientists suggested the name astatine from Greek astatos meaning unstable, the -ine ending used for halogens, and following the tradition that halogens are named after one of their properties (the longest-lived isotope of astatine has a half-life of 8 hours).
Astatine is the rarest element in the Earth’s crust and is highly unstable. As such, it has no applications.
Element 117 was discovered in a collaboration between the Joint Institute of Nuclear Research at Dubna, Lawrence Livermore National Laboratory in Livermore, California, Vanderbilt University, Tennessee, and Oak Ridge National Laboratory, Tennessee. A berkelium target was prepared in Oak Ridge and transported to Dubna where it was bombarded with calcium to produce six atoms of element 117 which were detected through their decay products. The results were announced in 2010. Further experiments between 2011 and 2014 confirmed the results of the earlier experiments and IUPAC recognised the discovery in 2015.
Amongst the collaborators, Joseph Hamilton of Vanderbilt University indicated he would choose the name as he had been instrumental in forming the collaboration and obtaining the berkelium target. The proposed name, tennessine, incorporated the -ine suffix was used for halogens and was adopted in 2016.
Tennessine has no applications as it is extremely expensive and difficult to produce and is highly unstable.
Matrix+ will give you access to HSC Chemistry Experts, wherever you are! Learn more.
Start HSC Chemistry confidently
Expert teachers, comprehensive resources, one-to-one help! Learn from home with Matrix+ Online.
© Matrix Education and www.matrix.edu.au, 2025. Unauthorised use and/or duplication of this material without express and written permission from this site’s author and/or owner is strictly prohibited. Excerpts and links may be used, provided that full and clear credit is given to Matrix Education and www.matrix.edu.au with appropriate and specific direction to the original content.