Welcome to Matrix Education
To ensure we are showing you the most relevant content, please select your location below.
Select a year to see courses
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Get HSC Trial exam ready in just a week
Get HSC exam ready in just a week
Select a year to see available courses
Science guides to help you get ahead
Science guides to help you get ahead

In this article, we give you the history and uses for the elements that are in the Actinoid Series.
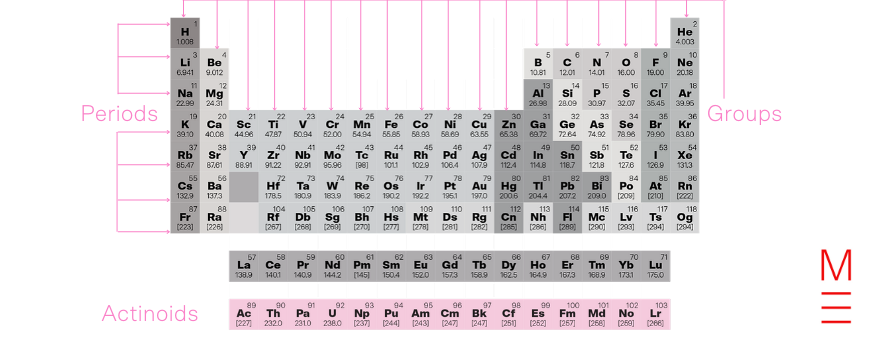
The actinoids are named after actinium, the first element in the series. They are defined by the progressive filling of the 5f orbital. They are all radioactive metals.
Together with the lanthanoids, they form the f-block.

All the key Chemistry concepts and formulas in one foldable document!

Fill out your details below to get this resource emailed to you.
"*" indicates required fields
Click on the following elements to learn more about them:
The discovery of actinium is contested. One possibility is that Andre-Louis Debierne discovered it in 1899 in the uranium mineral pitchblende. Marie and Pierre Curie had removed the uranium and radium from the pitchblende, and Debierne discovered an additional component in what remained that he claimed was similar to titanium. Subsequently he claimed it was similar to thorium. The second possibility is that Friedrich Oskar Giesel discovered it independently in 1902 and identified it as being similar to lanthanum. Debierne named the element actinium after the Greek actina meaning ray, and Giesel named it emanium, presumably both in reference to the radiation emanating from it. Debriene’s name was chosen, however in the 1970s, Debrierne’s results were questioned, and it was suggested that he mistook the then-unknown protactinium as actinium.
Actinium is extremely rare and has no applications, other than possible uses in radiation therapy. The longest-lived isotope has a half life of just under 23 years.
Although thorium is radioactive, its most stable isotope has an extremely long half-life of 14 billion years meaning it occurs naturally on Earth. In 1828, Morten Thrane Esmark discovered a mineral in Norway and sent it to his father Jens Esmark, a professor of mineralogy, who confirmed it was a new mineral. Jens Esmark sent the mineral to Jons Jacob Berzelius who determined that it contained a new metal. He isolated it and named it thorium, after the Norse god Thor, and named the mineral thorite. A few years prior to this, Berzelius had erroneously claimed the discovery of an element which he had named thorium and then retracted the claim. He reused the name in this subsequent (correct) discovery.
Thorium oxide was used in light sources (called thoriated gas mantles) as it remains solid and emits bright light when heated by a flame. Thorium was also used in alloys, ceramics and glass to improve their properties, and along with other radioactive substances, it was promoted as a cure for various diseases. Once the health effects of radiation were understood, its use in all applications was phased out.

Dmitri Mendeleev predicted the existence of protactinium, but the structure of the periodic table for heavier elements (i.e. the actinoid series) was not understood at the time and he incorrectly placed it under tantalum. Scientists therefore expected an element with similar chemical properties to tantalum, which they failed to find.
William Crookes was the first to isolate protactinium in 1900 but did not recognise it as a new element. In 1913, Kasimir Fajans and Oswald Helmuth Gohring discovered the element, and specifically the isotope protactinium-234 when studying the uranium-238 decay chain. They named it brevium after the Latin brevis meaning brief, as its half-life was 6.7 hours. Around five years later Otto Hahn and Lise Meitner and also Frederick Soddy and John Cranston independently discovered a different isotope, protactinium-231, when studying the uranium-235 decay chain. The half-life of this isotope was less brief – around 33,000 years – and Hahn and Meitner proposed the name protoactinium to indicated that it was the first before actinium in the uranium-235 decay chain (U-235 → Th-231 → Pa-231 → Ac-227 → …), from Greek proto meaning first. The name was shortened by IUPAC to protactinium.
Protactinium has no uses as it is very rare, highly radioactive and toxic.
Several uranium isotopes have sufficiently long half-lives (the longest being 4.5 billion years) that they are naturally found on Earth. Uranium compounds were used in Roman times as yellow pigments to colour ceramics and glass, and the uranium mineral pitchblende (mostly UO2) was used in medieval times in Europe for glassmaking. The element was discovered by Martin Heinrich Klaproth in 1789 when experimenting with pitchblende. He isolated a substance he believed to be a new metal and named it uranium. The name was in reference to the planet Uranus which had recently been discovered, itself named after Uranus, the sky god in Greek mythology. Klaproth had in fact produced a uranium oxide – metallic uranium was made in 1841 by Eugene-Melchior Peligot.
In 1896, Henri Becquerel discovered radioactivity when he placed uranium salts on unexposed photographic plates. He observed that the plates became exposed and concluded that uranium was emitting invisible radiation.
Uranium was used in glass, ceramics, dyes and certain alloys until the effects of radiation were understood and its use phased out. In the 1930s, uranium fission was discovered which led to the development of the nuclear industry. Uranium is now used to produce nuclear power, weapons and medicine as well as for counterweights, radiation shielding and ammunition. Its use is highly regulated.
The discovery of element 93 was complicated by an expectation that elements heavier than uranium would not exist naturally, a lack of understanding of nuclear physics, and the incorrect expectation that it would sit under rhenium in the periodic table which led to incorrect predictions about its chemical properties.
In 1934, Enrico Fermi suggested that element 93 was produced when he bombarded uranium with neutrons. He proposed the name ausonium, after the Greek Ausonia meaning Italy, as he was Italian. It was eventually understood that he was causing uranium to fission and not producing heavier elements. A claimed discovery in pitchblende – named bohemium – turned out to be a tungsten-vanadium mixture. Another discovery based on spectroscopy – named sequanium – was dismissed based on the belief that the element would not occur naturally.
In 1940, Yoshio Nishina carried out a similar neutron bombardment experiment and likely produced an isotope of element 93, however he failed to detect it. The extraction methods used assumed it behaved like rhenium, and the isotope he produced had a long half-life making it difficult to detect through the radiation emitted.
The element was discovered by Edwin McMillan and Philip Abelson. In 1939, McMillan detected an unknown half-life in a uranium target after bombardment, but failed to extract an element with the expected rhenium-like properties. In 1940, Abelson showed that an element with properties similar to uranium was present instead. They proposed the name neptunium, as the planet Neptune is the next one along after Uranus.
Neptunium’s main use is in the production of plutonium for the nuclear industry. The most stable isotope has a half-life of 2 million years.
In 1934, Enrico Fermi mistakenly claimed to have produced element 94 through neutron bombardment and named it hesperium, a Greek name for Italy, as he was Italian. Eventually it was understood that he was causing uranium fission and not producing heavier elements.
After the discovery of neptunium, it was realised that neptunium would beta decay to form element 94, which was isolated in 1941 by Glenn Seaborg, Edwin McMillan, Emilio Segre, Joseph Kennedy and Arthur Wahl at the University of California Berkeley. The result was not announced until 1946 due to security concerns surrounding World War II. McMillan (who had discovered and named neptunium) proposed the name plutonium after Pluto, the next then-planet along. Other names considered were plutium, ultimium or extremium, the latter two being an erroneous reference to it being the heaviest element possible. The symbol Pu was proposed as a joke (a reference to a bad smell), but was adopted.
Plutonium is produced for use in nuclear reactors and weapons. One isotope is used in thermoelectric generators to power spacecraft, and initially to also power pacemakers. The most stable isotope has a half-life of 80 million years.
Americium was first produced in 1944 by Glenn Seaborg, Leon Morgan, Ralph James, and Albert Ghiorso at the University of California Berkeley, and identified at the University of Chicago. The work was part of the Manhattan Project, so the result was kept secret until late 1945. Its position in the periodic table is below europium (named after Europe), so it was named americium (after the Americas) by analogy. There are no stable isotopes of americium. The two most stable isotopes have half-lives of 7,370 and 432 years, and most commonly alpha decay to neptunium.
Americium can be extracted from spent fuel rods from nuclear reactors and is used as a radiation source. The most familiar application is in ionisation smoke detectors.

Curium was fabricated at the University of California Berkeley in 1944 by Glenn Seaborg, Ralph James and Albert Ghiorso, and subsequently isolated chemically at the University of Chicago. They produced it by bombarding recently discovered plutonium (element 94) with alpha particles, thereby adding two protons to each nucleus. The discovery remained classified due to the Manhattan Project until 1945. The element sits below gadolinium in the periodic table, named after Gadolin to recognise his work on lanthanoids. By analogy, they named the element curium after Marie and Pierre Curie to recognise their work on radioactivity.
Curium has very few niche research applications, such as radiation sources on spacecraft and the production of heavier elements. The most stable isotope has a half-life of 15.6 million years.
Glenn Seaborg, Albert Ghiorso, Stanley Thompson, and Kenneth Street Jr produced element 97 in 1949 at the University of California Berkeley. They bombarded americium (element 95) with alpha particles, adding two protons to each nucleus. The element sits below terbium in the periodic table, named after Ytterby in Sweden where minerals containing terbium were first found. By analogy they named the new element Berkelium after Berkeley in California, where this element was first synthesised.
There are no uses for berkelium other than in heavy element research. The most stable isotope has a half-life of 1380 years.
Californium was produced in 1950 by Stanley Thomson, Kenneth Street Jr, Alber Ghirso, and Glenn Seaborg at the University of California Berkeley. Similar to the discovery of previous heavy elements, they bombarded curium (element 96) with alpha particles to add two protons to the nucleus and create element 98. They named it californium after their university and state.
Californium is used as a neutron source for nuclear reactors, for the characterisation of materials, and in some very specific cancer treatments. The most stable isotope has a half-life of 898 years.
Einsteinium was discovered in 1952 from Ivy Mike nuclear weapons test, on filter paper that was flown through the explosion cloud to sample the fallout. The discovery was made at the University of California Berkeley by a team headed by Albert Ghiorso. The specific conditions of the explosion resulted in uranium-238 atoms absorbing 15 neutrons and then beta decaying seven times to produce element 99. This discovery was classified until 1955, but the element was synthesised in the lab at Berkeley and Argonne Laboratories and these later results were published in 1954. The element was named in 1955 after the recently deceased Albert Einstein in recognition of his contribution to physics. The proposed symbol E was changed to Es by IUPAC.
Einsteinium has no applications as it is extremely expensive and difficult to produce and is highly unstable.
Fermium was discovered in 1952 from Ivy Mike nuclear weapons test, by processing contaminated coral from the atoll in which the bomb had been detonated. The discovery was made at the University of California Berkeley by a team headed by Albert Ghiorso. The specific conditions of the explosion resulted in uranium-238 atoms absorbing 17 neutrons and then beta decaying eight times to produce element 100. This discovery was classified until 1955, but the element was synthesised in the lab and these later results were published in 1954. The element was named in 1955 after the recently deceased Enrico Fermi in recognition of his contribution to nuclear physics, specifically his development of the first nuclear reactor.
Fermium has no applications as it is extremely expensive and difficult to produce and is highly unstable.
Mendelevium was first synthesized in 1955 at the Lawrence Berkeley National Laboratory, University of California Berkeley. They bombarded einsteinium (element 99) with alpha particles to add two protons to the nucleus and create element 101. The element was confirmed indirectly – by observing the fission of fermium-256, which could have only been present if element 101 decayed to fermium. The element was named after Dmitri Mendeleev, the father of the periodic table.
Mendelevium has no applications as it is extremely expensive and difficult to produce and is highly unstable.
In 1957, the Nobel Institute in Sweden reported the fabrication of element 102 by bombarding curium atoms with carbon ions. They detected specific radiation from their samples which they attributed to element 102. The name nobelium, after Alfred Nobel, was proposed and accepted by IUPAC. However, in 1958, the experiment was repeated at the Lawrence Berkeley National Laboratory and the specific radiation noted by the Swedish group was not detected. The Swedish group eventually retracted their claim.
In 1958 and 1959, the Berkely group claimed to have produced element 102 using a similar method to the Swedes, but their results were not fully understood. At the same time, a team at the Joint Institute for Nuclear Research in Dubna attempted to bombard plutonium with oxygen, but also produced inconclusive results. This was followed by two more claims from Dubna using uranium and neon, in 1964 and 1966. The Dubna team proposed the named joliotium after the recently deceased Irene Joliot-Curie, while the Berkeley team continued to call the element nobelium.
In 1992, IUPAC recognised the 1966 Dubna result as the discovery of element 102 and verified the name nobelium as it was already established at the time. They changed the name to flerovium in 1995 after objections, and then back to nobelium in 1997 after further objections.
Nobelium has no applications as it is extremely expensive and difficult to produce and is highly unstable.

In 1961, the Lawrence Berkeley National Laboratory bombarded californium atoms with boron and claimed to have detected element 103. They proposed the name lawrencium and the symbol Lw after Ernest Lawrence, the inventor of the cyclotron which had been used in previous bombardment experiments. IUPAC accepted the name and changed the symbol to Lr, however the results were questioned. In 1965 the Joint Institute for Nuclear Research in Dubna bombarded americium with oxygen and also claimed to have discovered element 103, which they named rutherfordium. Further experiments at Berkeley and Dubna continued until 1977 by which time several isotopes of element 103 were characterised.
In 1971, IUPAC recognised the Berkeley group as the discoverers, however this was retracted in 1992 when both Dubna and Berkeley were recognised as co-discoverers. The name lawrencium was ratified in 1997 as it was already established.
Lawrencium has no applications as it is extremely expensive and difficult to produce and is highly unstable.
Matrix+ will give you access to HSC Chemistry Experts, wherever you are! Learn more.
Start HSC Chemistry confidently
Expert teachers, comprehensive resources, one-to-one help! Learn from home with Matrix+ online.
© Matrix Education and www.matrix.edu.au, 2025. Unauthorised use and/or duplication of this material without express and written permission from this site’s author and/or owner is strictly prohibited. Excerpts and links may be used, provided that full and clear credit is given to Matrix Education and www.matrix.edu.au with appropriate and specific direction to the original content.