Welcome to Matrix Education
To ensure we are showing you the most relevant content, please select your location below.
Select a year to see courses
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Get HSC Trial exam ready in just a week
Get HSC exam ready in just a week
Select a year to see available courses
Science guides to help you get ahead
Science guides to help you get ahead
In this article, we give you the history and uses for the elements in Group 18 – Noble Gases.
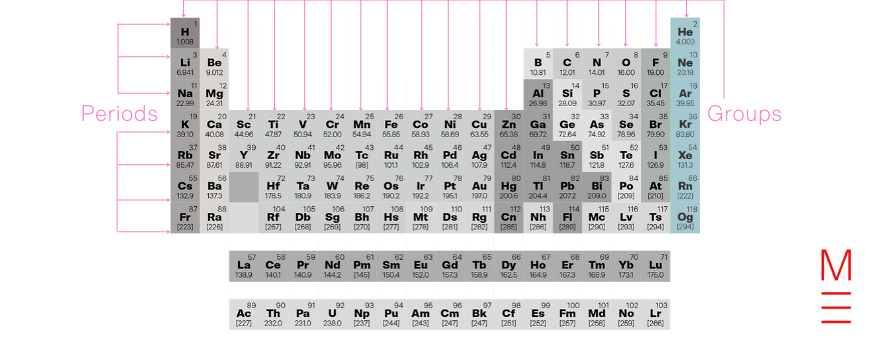
Group 18 is the noble gases. They have a full outer shell which makes them stable – as a result, these don’t react.
Together with groups 13-17, they form the p-block.

All the key Chemistry concepts and formulas in one foldable document!

Fill out your details below to get this resource emailed to you.
"*" indicates required fields
Click on the following elements to learn more about them:
Helium was first discovered when distinct spectral lines were observed in the Sun by Jules Janssen in 1868, however the lines were mistakenly believed to come from sodium. Later in 1868 Norman Lockyer confirmed the observation and concluded it was due to a new element that he and Edward Frankland named helium after the Greek helios, meaning the Sun. In 1881, Luigi Palmieri detected helium on Earth through the same spectroscopic methods when analysing material from a volcanic explosion.
The main use of helium is in cryogenic applications for cooling superconducting magnets used, e.g. in MRI scanners. More familiar applications include helium balloons and blimps.
Neon was discovered by Sir William Ramsay and Morris Travers in 1898 as a component of air. They liquified air and slowly increased the temperature to allow different gases to boil off at their respective boiling points. Neon was distinct in producing a bright red-orange light when electricity is passed through it in a discharge tube. The name novum was suggested, meaning new in Latin, however neon was chosen, meaning new in Greek.
The most common use of neon is in the distinctive red-orange neon light signs.
Argon was discovered by Lord Rayleigh and Sir William Ramsay in air, by removing other known gases through absorption into other chemicals or by reacting them. They failed to reconcile the masses of the chemicals produced and concluded that a small portion of the air remained unreacted and must have been composed of a new inert gas. They named the gas argon after the Greek argon meaning inactive. The symbol A was used for the element until 1957 when it was changed to Ar.
Argon is most commonly used as an inert atmosphere as it is the most abundant noble gas, and hence the cheapest to produce.
Krypton was discovered by William Ramsay and Morris Tavers in an experiment where they liquified air and then evaporated its various components. They named it after the Greek kryptos meaning hidden.
Krypton is used in a variety of different lamps, as well as krypton fluoride lasers. One of the krypton emission lines was used to define the metre between 1960 and 1983.
In 1898, William Ramsay and Morris Travers discovered xenon by cooling down air to form a liquid and then evaporating off known components (they had discovered neon and krypton in the same way). They named it after the Greek xenos meaning foreign.
The main use of xenon is in lamps, particularly in high brightness lamps like photographic flashes, projectors and headlights.
In 1899, Ernest Rutherford and Robert Owens noted that thorium emits a radioactive gas which they initially named emanation, and subsequently named thorium emanation. In the next few years similar observations were made using radium and actinium: the Curies and, separately, Friedrich Ernst Dorn discovered radium emanation, and Andre-Louis Debierne discovered actinium emanation. These names were eventually condensed into thoron (To), radon (Ro) and actinon (Ao).
In 1904, Sir William Ramsay used spectroscopy to suggest that the emanations were a new noble gas, and in 1909, he and Robert Whytlaw-Gray characterised its properties and suggested the name niton (Nt), from Latin meaning shining, a reference to radioluminescence. The three emanations were in fact three isotopes of the same element, with radon, thoron and actinon having different mass numbers of 222, 220 and 219 respectively. In the end, IUPAC chose the name radon for the element, and the symbol Rn, as radon is the most stable of the three isotopes (half life of 3.8 days). The name change led to confusion about the discovery – Rutherford and Owens discovered the element radon, whilst Dorn discovered the isotope originally called radon.
Radon is radioactive and poses a health hazard if inhaled. It is sometimes used in cancer therapy and other medical applications.
Radium gas in vial. The vial contains a very small amount of thorium, which is in equilibrium with its decay products. One of these is radon-220 (the isotone originally called thoron), which alpha decays with a half-life of 55.6 seconds. The activity of the radon gas in the sample is 0.58 kBq and the glass vial blocks the alpha radiation produced. For comparison, a human body has an activity of around 8 kBq.
In 1999, the Lawrence Berkeley National Laboratory claimed to have fabricated element 118 by bombarding lead with krypton. They intended to propose the name ghiorsium after Albert Ghiorso, a prominent member of the Berkeley team who had contributed to the discovery of 12 elements. However, neither they nor others could replicate the results and the claim was retracted in 2001. It was eventually realised that the 1999 results had been fabricated.
In 2002, a collaboration between the Joint Institute of Nuclear Research at Dubna and Lawrence Livermore National Laboratory believed they produced one or two atoms of element 118 by bombarding californium with calcium. They attempted to verify the results in 2005, producing two more atoms, before announcing the result in 2006. In 2011, IUPAC deemed the results insufficient, but recognised the discovery in 2015 after further work was done to verify the properties of the decay products used to detect the presence of the element.
The Dubna group intended the names flyorium or moskovium, after Georgy Flyorov, founder of the Dubna facility and the Moscow Oblast in which it is located, however those names were used for other elements. In 2016 the Dubna-Livermore collaboration unanimously proposed the name oganesson after Yuri Oganessian, the director of the Flerov Laboratory of Nuclear Reactions at the Joint Institute for Nuclear Research. This was in recognition of his 60 year career in heavy ion research, and his contribution to the discovery of elements 107 through to 118. After a minor disagreement with IUPAC about spelling, oganesson was made official in the same year.
Oganesson has no applications as it is extremely expensive and difficult to produce and is highly unstable.
Matrix+ will give you access to HSC Chemistry Experts, wherever you are! Learn more.
Start HSC Chemistry confidently
Expert teachers, comprehensive resources, one-to-one help! Learn from home with Matrix+ Online.
© Matrix Education and www.matrix.edu.au, 2025. Unauthorised use and/or duplication of this material without express and written permission from this site’s author and/or owner is strictly prohibited. Excerpts and links may be used, provided that full and clear credit is given to Matrix Education and www.matrix.edu.au with appropriate and specific direction to the original content.