Welcome to Matrix Education
To ensure we are showing you the most relevant content, please select your location below.
Select a year to see courses
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Select a year to see available courses
Science guides to help you get ahead
Science guides to help you get ahead
What is the Periodic Table and why does it matter?
The periodic table is a chart that shows all the known chemical elements in one place. It helps students and scientists quickly understand how different elements are related based on their atomic structure.
It’s organised by patterns — such as how many protons and electrons atoms have — so that elements with similar properties line up. That’s why it’s called “periodic.”
In this guide, we’ll break it all down so you understand what the table shows, how to read it, and why it’s useful in Chemistry.
The periodic table of the elements (or just the periodic table) is a table that organises all of the chemical elements.
There are 118 known elements (at this point), so it is important that they be organised conveniently and sensibly. The periodic table does this by sorting elements into periods and groups according to the number of electron shells and their electronic configuration.
Each element has a unique atomic number, which is the number of protons in its nucleus. As you move across the table from left to right, the atomic number increases.
The periodic table is structured into:
Periods – the rows going across
Groups – the columns going down
Elements in the same group often react in similar ways because they have the same number of outer electrons.
An element is an atom that has a specific number of protons (a subatomic particle with a positive charge +) in its nucleus. We use the number of protons in the nucleus to identify the element, and call this its atomic number (Z). For example:
Hydrogen has 1 proton
Helium has 2 protons
Carbon has 6 protons
Most substances we find on Earth are made of compounds, which are combinations of elements. However, a few elements are found in pure form, like gold.
The discovery and naming of elements is now overseen by the International Union of Pure and Applied Chemistry (IUPAC). Research into new elements is an ongoing process.
A period is a horizontal row on the periodic table of elements.
Each element in a period has the same number of electron shells. (An electron shell is an orbit that an electron follows around the nucleus of the atom.)
As you move from left to right across a period, each element has one more proton in its nucleus compared to the preceding one. The properties also change across a period.
This arrangement explains why:
Metals are typically found on the left
Non-metals are on the right
The elements gradually change in reactivity and other properties
This repeating pattern is known as the Periodic Law — it means that certain chemical properties reoccur at regular intervals. When a period ends, the next element begins a new row with similar characteristics to the element directly above it.
This is why each column ends up containing elements that have similar properties – each column is called a group.
A group is a column of elements in the periodic table. Each table of elements group contains elements with similar physical or chemical characteristics, which mostly arise from the number of electrons in their outermost electron shell (called the valence shell).
There are 18 groups, numbered from left to right. Some of these groups have quite different properties from others, like the noble gases, but some share similar properties, like the transition metals, and are sometimes considered together.
Key groups in the periodic table are:
| Groups | Number of elements | |
| 1 | Group 1 (Hydrogen and alkali metals) | 7 |
| 2 | Group 2 (Alkaline earth metals) | 6 |
| 3 | Groups 3-12 (Transition metals) | 38 |
| 4 | Groups 13-16 | 24 |
| 5 | Group 17 (Halides) | 6 |
| 6 | Group 18 (Noble gases) | 7 |
| 7 | Lanthanoids | 15 |
| 8 | Actinoids | 15 |
The modern periodic table was created by Dmitri Mendeleev in 1869, but he wasn’t the first to try organising the elements.
Back in 1789, French chemist Antoine Lavoisier grouped elements into basic categories like gases, metals, nonmetals, and earths.
In 1862, Alexandre-Émile Béguyer de Chancourtois noticed that element properties repeated in patterns. He arranged them by atomic weight into a spiral (helix) shape, laying the groundwork for the idea of periodicity.
Later, John Newlands introduced the Law of Octaves in which every eighth element shared similar properties—much like notes in music. This helped predict where undiscovered elements would go.
Finally, in 1864, German chemist Julius Lothar Meyer grouped elements by valence, which explains how elements bond. His work helped form the basis for the table of elements groups we use today.
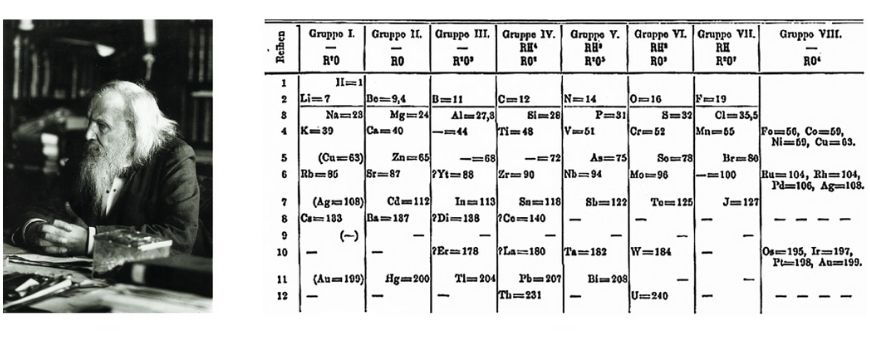
Finally, Mendeleev’s 1869 table placed elements by atomic weight and left intentional gaps for undiscovered elements. His predictions proved correct, solidifying the table’s usefulness.
Back then, scientists didn’t yet understand atomic structure — like protons, electrons, or electron shells. It wasn’t until the 1950s that the table was reorganised based on atomic number and electronic configuration, giving us the version we use today.
To help you use this Guide, we’ve incorporated the following elements (pun intended!):
Use the chapter titles to navigate through the various groups of elements.
Each chapter of this guide has a contents table to help you navigate quickly to each element.
Use the information to reinforce your knowledge and if you’re at Strathfield campus, check out our Periodic Table Display.
The Matrix Strathfield Campus has a Periodic Table display with samples of all the common elements (sorry, we don’t have a particle accelerator to create exotic elements!). We’re pretty proud of it, so you should check it out if you’re on campus.
The periodic table is a chart that organises all known chemical elements based on their atomic number, electron configuration, and recurring properties. It is structured into horizontal rows (periods) and vertical columns (groups).
An element is a substance made up of atoms that all have the same number of protons in their nuclei. Each element has a unique atomic number.
A period is a row on the periodic table. Elements in the same period share the same number of electron shells, and their properties change gradually across the row.
A group is a column of elements on the periodic table. Elements in the same group have similar properties because they have the same number of electrons in their outer shell (valence electrons).
The modern periodic table was created by Dmitri Mendeleev in 1869. Earlier attempts were made, but Mendeleev’s version organised elements by atomic weight and predicted new elements. Its structure was later refined using atomic number.
© Matrix Education and www.matrix.edu.au, 2025. Unauthorised use and/or duplication of this material without express and written permission from this site’s author and/or owner is strictly prohibited. Excerpts and links may be used, provided that full and clear credit is given to Matrix Education and www.matrix.edu.au with appropriate and specific direction to the original content.