Welcome to Matrix Education
To ensure we are showing you the most relevant content, please select your location below.
Select a year to see courses
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Select a year to see available courses
Science guides to help you get ahead
Science guides to help you get ahead
Want to see how you went in HSC Chemistry? Or working through past papers? Here are the 2025 HSC Chemistry Exam Paper Solutions to help you.
Join 75,893 students who already have a head start.
"*" indicates required fields
You might also like
Related courses

Join 8000+ students each term who already have a head start on their school academic journey.
Check your answers to the 2025 Chemistry HSC. Our Chemistry team has written complete, step-by-step solutions to the 2025 HSC Chemistry exam. Use this page to see where you picked up marks and how to improve for trials and beyond.
These are the responses to the 2025 Chemistry paper, which will be linked here on the NESA website once available.
You can also find a list of all the latest HSC Chemistry Exam Paper Solutions here.
| Question | Answer | Explanation |
| 1 | B | A strong acid is completely ionised in water, so the solution will not contain intact acid HA. The diagram only shows H+ and A− ions, indicating a strong acid is illustrated. A concentrated solution contains a large number of moles of acid per volume. Only a few ions are illustrated, suggesting solution is not concentrated. |
| 2 | B | The reaction scheme shows conversion of a secondary alcohol into a ketone. This type of reaction is oxidation. |
| 3 | A | 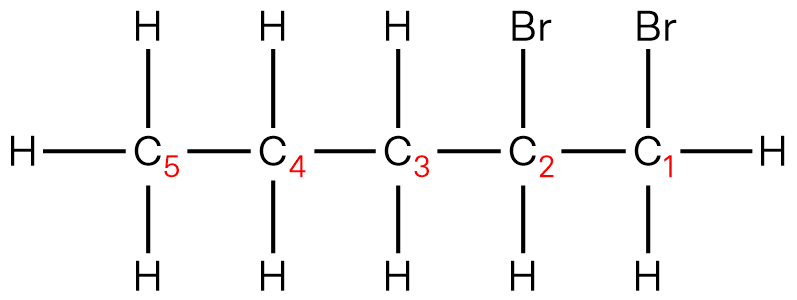 |
| 4 | A | I− ions react with Pb2+ ions to form yellow lead(II) iodide precipitate but not with Na+ ions. Ammonium, nitrate and acetate ions do not form any precipitate with either Pb2+ or Na+. |
| 5 | C | Since only PCl3 and Cl2 are introduced into the vessel, PCl3 and Cl2 will combine to form PCl5. This means the concentration of Cl2 is initially non-zero and will decrease as the reaction progresses. This eliminates options B and D. As the vessel is closed and the reaction is reversible, the system will reach dynamic equilibrium. This means the concentration of Cl2 will eventually be a constant non-zero value. |
| 6 | D | [H3O+] = [HCl] = 0.25 mol L−1 pH = −log10[H3O+] = −log10(0.25) = 0.60 (2 s.f.) |
| 7 | C | The two complexes are different colours, hence would absorb different wavelengths of visible light. The unique wavelengths absorbed can be measured using UV-visible spectrophotometry. |
| 8 | D | When an alkene undergoes an addition reaction, the double bond breaks open to allow atoms to be added, without the elimination of any atoms. Hence the difference in molar mass between Y and X corresponds to the molar mass of the unknown substance added. |
| 9 | A | The water-soluble carbohydrate chain and fat-soluble side chain of saponin allows it to function as a surfactant. The water-soluble region allows it to interact with water while the fat-soluble region allows it to interact with non-polar grease and oil. |
| 10 | D | Butanoic acid is a covalent molecule, and all covalent molecules exhibit dispersion forces. The carboxyl functional group makes butanoic acid polar, hence butanoic acid will also be able to form dipole-dipole forces. The hydrogen atom bonded to oxygen in the carboxyl functional group allows hydrogen bonding to form with the lone pair of electrons on the oxygen. Covalent bonds are intramolecular bonds, which means they exist between atoms within a molecule, not between molecules. |
| 11 | A | A Brønsted-Lowry acid is a proton donor and a Brønsted-Lowry base is a proton acceptor. X is the conjugate base of propanoic acid (a weak acid). Hence it can accept a proton to produce propanoic acid. 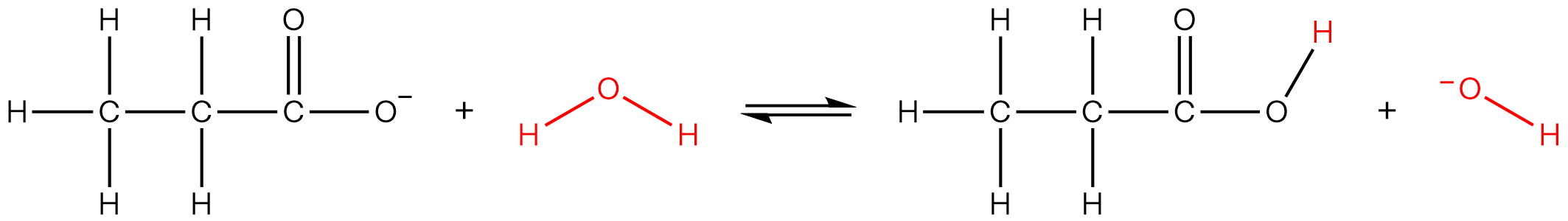 Y is ethanamine, a weak organic base. It can accept a proton to form ammonium ion. 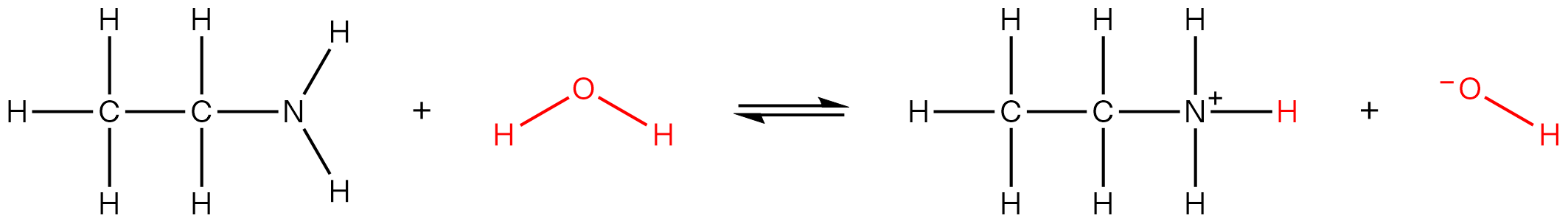 Hence both X and Y are Brønsted-Lowry bases. |
| 12 | B | The equilibrium expression is given by: The concentration of a species is raised to the power of its respective stoichiometric coefficient in the equation. |
| 13 | C | Combustion of octane releases heat energy, hence the reaction is exothermic and enthalpy decreases (i.e., ΔH < 0). The combustion of octane at 100 °C is: C8H18(l) + 25/2 O2(g) → 8CO2(g) + 9H2O(g) There are more gaseous particles as products form, hence entropy increases (i.e., ΔS > 0). |
| 14 | B | 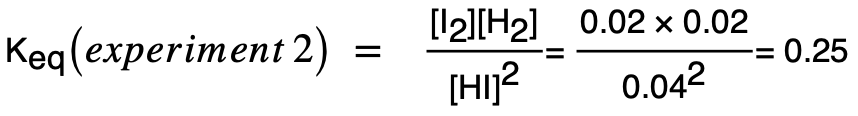 Hence, Keq is lower in experiment 1. A larger Keq in experiment 2 means more products are present and equilibrium has shifted in the forward endothermic direction compared to reaction 1. |
| 15 | D | The first reaction in the sequence is a hydrogenation, which involves breaking of the carbon-carbon double bond to allow the addition of H2. The −OH group does not participate in the reaction so is unchanged. This gives product X as propan-1-ol. The second reaction in the sequence is oxidation. Since propan-1-ol is a primary alcohol, the oxidation product (Y) can be propanal or propanoic acid. However, since the question states Y forms bubbles of gas with aqueous sodium carbonate, it must be propanoic acid, as carboxylic acids react with metal carbonates to form carbon dioxide gas. The last reaction in the sequence is an esterification. Reaction of propanoic acid and methanol gives product Z as methyl propanoate. |
| 16 | B | The polymerisation reaction results in elimination of n − 1 H2O molecules, where n = number of monomer units. Since 1000 monomer units joined together, 999 water molecules are eliminated. Hence MM(polymer) = MM(monomer) × 1000 − MM(H2O) × 999 = (12.01 × 3 + 1.008 × 6 + 16.00 × 3) × 1000 − (1.008 × 2 + 16.00) × 999 = 72080.016 g mol−1 Alternative: Polymer structure is H—(—OCH2CH2CO—)1000—OH Hence MM(polymer) = (12.01 × 3 + 1.008 × 4 + 16.00 × 2) × 2 + (1.008 × 2 + 16.00) = 72080.016 g mol−1 |
| 17 | A | 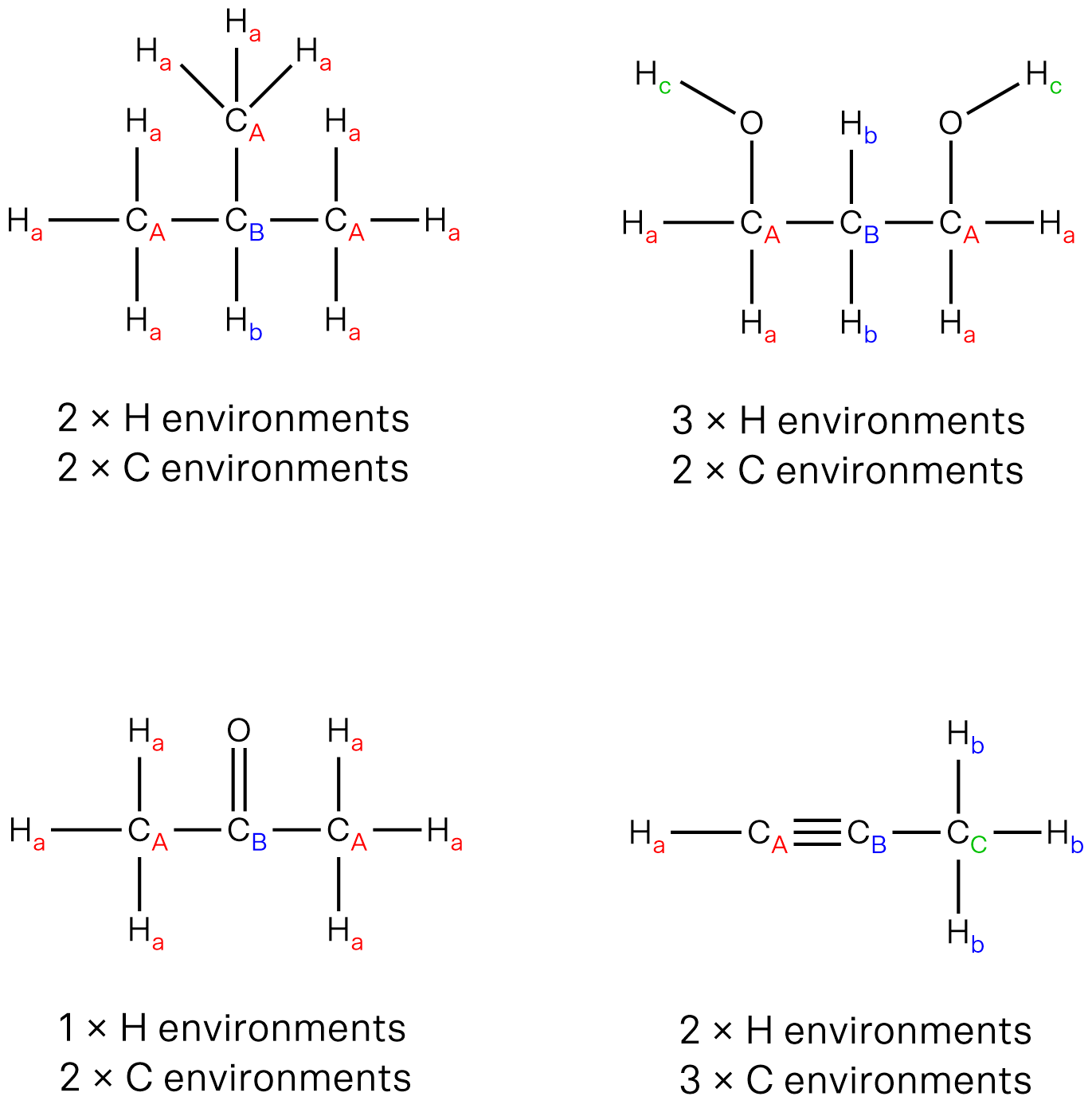 |
| 18 | A | The question states silver ions are titrated with aqueous sodium chloride, implying sodium chloride solution is the titrant added and silver ions are initially present in the flask with potassium chromate indicator. Silver ions precipitate with chromate ions to form red silver chromate precipitate. Hence the solution will initially be red. 2Ag+(aq) + CrO42−(aq) ⇌ Ag2CrO4(s) Silver ions can also precipitate with chloride ions. Ag+(aq) + Cl−(aq) ⇌ AgCl(s) However, as silver chloride is more insoluble than silver chromate, the addition of chloride ions causes silver chromate to dissociate to produce silver ions. As silver ions continue to precipitate with added chloride ions, all the red silver chromate will dissociate. This leaves behind yellow chromate ions, resulting in the solution changing from red to yellow. |
| 19 | C | Sodium acetate dissociates to give acetate ions, which undergo neutralisation with HCl. CH3COO−(aq) + HCl(aq) → CH3COOH(aq) + Cl−(aq) n(HCl)initial = c × V = 0.1 × 0.500 = 0.05 mol n(CH3COO−)initial = 0.1 mol HCl is the limiting reactant. After neutralisation, 0.05 mol of CH3COO− remain and 0.05 mol of CH3COOH forms. The following equilibrium between acetic acid and acetate ions exists in solution: CH3COOH(aq) + H2O(l) ⇌ CH3COO−(aq) + H3O+(aq) Since equal moles CH3COOH and CH3COO− are present, a buffer solution will be present. The Henderson–Hasselbalch equation can be used to approximate the pH of a buffer solution: pH = pKa + log10([A−]/[HA]) The concentrations immediately after neutralisation are: [CH3COOH] and [CH3COO−] = 0.05 / 0.5 = 0.1 mol L−1 The concentrations after dilution are: [CH3COOH] and [CH3COO−] = 0.05 / 0.1 = 0.5 mol L−1 (note: adjust if your intended final volume is 0.1 L; if not, keep your original figure) When [HA] and [A−] are equal, log10([A−]/[HA]) = 0, and pH = pKa. Hence, the pH remains at 4.8.Calculation proof: [H3O+]equilibrium = 10−4.8 mol L−1 Ka = [CH3COO−][H3O+] / [CH3COOH] = (0.1 + 10−4.8)(10−4.8)/(0.1 − 10−4.8) = 1.5853956 × 10−5 Then considering dilution:Since Ka is small, assume change in concentration of CH3COOH and CH3COO− is small. Ka = (0.05)(x)/(0.05) = 1.5853956 × 10−5 x = 1.5853956 × 10−5 = [H3O+] pH = −log10[H3O+] = −log10(1.5853956 × 10−5) = 4.8 |
| 20 | B | Ag2C2O4(s) ⇌ 2Ag+(aq) + C2O42−(aq) Ksp = [Ag+]2[C2O42−] [Ag+]dilute = 0.11 × 10−6 mol L−1 [Ag+]saturated = 2000 × 0.11 × 10−6 = 2.2 × 10−4 mol L−1 [C2O42−] = ½ × 2.2 × 10−4 Ksp = (2.2 × 10−4)2 × (½ × 2.2 × 10−4) = 5.324 × 10−12 |
Reaction condition X: UV light
IUPAC name of organic product: 2-bromobutane
Both qualitative and quantitative analyses are essential for assessing water quality, as they offer complementary insights.
Qualitative analysis identifies which chemical species are present in a water sample, such as toxic heavy metals. This helps to quickly determine if there is potential contamination of the water and whether further quantitative testing or remediation is necessary.
Quantitative analysis measures how much of a chemical species is present. This allows comparison with environmental or health standards (e.g., drinking water guidelines) to evaluate whether contaminant levels are within safe limits or pose potential risks.
Filter mixture after step 3, before adding BaCl2. There may be insoluble components in fertiliser which inflate the measured mass of the precipitate.
Dry the precipitate until constant mass is achieved, instead of just 20 minutes in the sun. Twenty minutes may not be sufficient for all the water to evaporate. The water will inflate the measured mass of the precipitate.
Other answers could include:
Structural formula:
Shape: Linear
n(C2H2) = m / MM = 65.0 / 26.04 = 2.496159754 mol
n(C2H3Cl) = n(C2H2) = 2.496159754 mol (1:1)
m(C2H3Cl) = n × MM = 2.496159754 × 62.50 = 156.00998 g = 156 g (3 s.f.)
Since propan-1-ol and butanoic acid react in a 1:1 ratio, the smaller moles of propan-1-ol makes it the limiting reactant.
n(ester)theoretical = n(propan-1-ol) = 0.267 mol (1:1)
m(ester)theoretical = n × MM = 0.267 × 130.2 = 34.7634 g
m(ester)actual = d × V = 12.2 × 0.873 = 10.6506 g
%yield = (m(ester)actual / m(ester)theoretical) × 100 = (10.6506 / 34.7634) × 100 = 30.6% (3 s.f.)
As the halogen in the haloethanoic acid varies down Group 17, the pKa increases.
Since pKa = −log10Ka, a larger pKa corresponds to a smaller Ka.
Ka = [X−CH2COO−][H3O+] / [X−CH2COOH]. Hence a smaller Ka indicates a less ionised acid.
Thus, as the halogen in the haloethanoic acid varies down Group 17, the strength of the acid decreases.
The combustion of petrol obtained from crude oil can result in the formation of SO2(g). This is a result of the oxidation of sulfur impurities found in crude oil. Sulfur dioxide can be further oxidised to sulfur trioxide: 2SO2(g) + O2(g) ⇌ 2SO3(g). Sulfur trioxide can react with water in the atmosphere to form sulfuric acid, which falls to the earth as acid rain. Acid rain can leach metals such as aluminium from soil, causing harm to aquatic life.
Other answers can include:
Combustion of petrol can release toxic products such as CO(g) or C(s) when insufficient oxygen is supplied.
Combustion of petrol releases CO2(g) into the atmosphere, which contributes to global warming.
The polar amide linkages in Kevlar chains enable the formation of strong hydrogen bonds between adjacent chains. In contrast, polystyrene chains are non-polar and interact only through dispersion forces.
As a result, the intermolecular forces between Kevlar chains are much stronger and require more energy to overcome than those in polystyrene. This makes Kevlar more difficult to pull apart, giving it high strength, hardness, and a high melting point.
Polystyrene, on the other hand, is relatively softer, has lower tensile strength, and melts at a lower temperature. Furthermore, the bulky benzene side groups in polystyrene restrict chain flexibility, contributing to its brittleness.
The addition of an inert gas such as argon will not disturb the equilibrium of the reaction. This is because the addition of an inert gas will not change the partial pressure of NO2(g) and N2O4(g) as the volume is fixed; as such there is no change in the reaction quotient, hence Q = K as the reaction is initially at equilibrium.
Since the system is still at equilibrium, the rate of the forward exothermic reaction is equal to the rate of the reverse endothermic reaction. Therefore, there will be no change in temperature in the system as the heat produced in the forward reaction will be absorbed by the endothermic reverse reaction.
Since phosgene is a highly toxic gas, it should be handled in a certified fume hood that can capture and remove phosgene vapour at the source, preventing it from accumulating in the breathing zone and throughout the lab.
Respiratory protection such as a properly fitted full-face respirator can be worn to provide additional protection against inhalation of vapours.
Excess carbon monoxide gas is used to increase the yield of phosgene. A higher concentration of carbon monoxide gas in the system disturbs the equilibrium. According to Le Chatelier’s principle, the equilibrium shifts in the forward direction to remove excess carbon monoxide and to minimise the disturbance. As a result, more phosgene is produced.
A catalyst is used to increase the rate of phosgene production. A catalyst provides an alternate reaction pathway of lower activation energy; hence a greater proportion of collisions can exceed the activation energy and be successful.
Suppose the molecular formula of hydrazine is N2H4. The complete combustion equation would be: N2H4(g) + 3O2(g) → 2NO2(g) + 2H2O(g).
Using Avogadro’s Law, which states that the volume of gas is proportional to the number of moles of gas present at a constant temperature and pressure, we get the stoichiometric ratio of n(hydrazine):n(O2):n(NO2):n(H2O) = 1:3:2:2 which matches the volumes of gas provided 1 L: 3 L: 2 L: 2 L.
| Concentration (mol L−1) | N2H5+(aq) | N2H4(aq) | H3O+(aq) |
| Initial | 0.20 | 0 | 0 |
| Change | −x | + x | + x |
| Equilibrium | 0.20 − x | x | x |
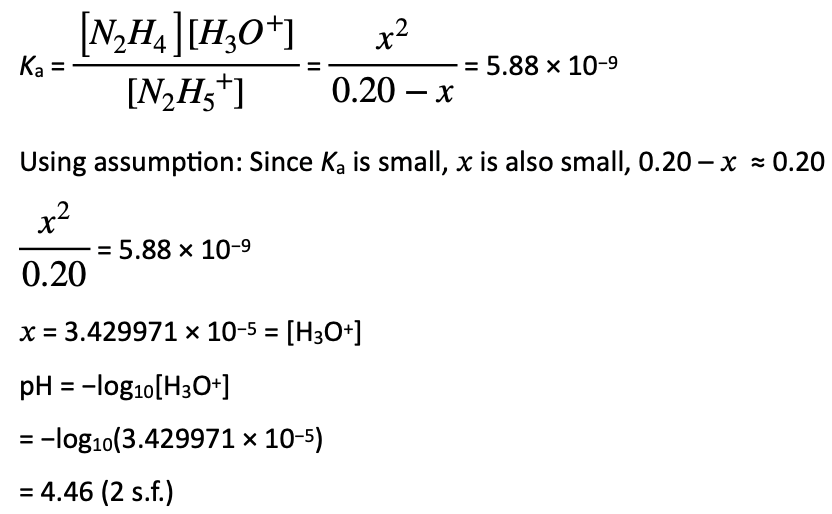
Ions present are Mg2+, Na+, NO3−, OH−, CO32−. Na+ and NO3− are always soluble so won’t form precipitates. Thus, possible precipitates are Mg(OH)2(s) and MgCO3(s).
Mg(OH)2(s) ⇌ Mg2+(aq) + 2OH−(aq) Ksp = [Mg2+][OH−]2 = 5.61 × 10−12
MgCO3(s) ⇌ Mg2+(aq) + CO32−(aq) Ksp = [Mg2+][CO32−] = 6.82 × 10−6
Since Ksp of Mg(OH)2 is significantly smaller than Ksp of MgCO3, Mg(OH)2 is less soluble and would precipitate out before MgCO3.
[Mg2+] = 0.006 mol L−1
[OH−] = 0.010 mol L−1
Q(Mg(OH)2) = [Mg2+][OH−]2 = 0.006 × (0.010)2 = 6 × 10−7 > Ksp(Mg(OH)2)
Hence Mg(OH)2 will precipitate.
Assuming precipitation of Mg(OH)2 goes to completion: Mg2+(aq) + 2OH−(aq) → Mg(OH)2(s)
n(Mg2+)initial = 0.006 mol
n(OH−)initial = 0.010 mol
OH− is the limiting reactant and Mg2+ is in excess
n(Mg2+) remaining = 0.006 − 0.005 = 0.001 mol
[Mg2+] remaining = 0.001 mol L−1
Since Ksp of Mg(OH)2 is small and there is excess common ion Mg2+ in solution, we can assume concentration of Mg2+ does not change.
[CO32−] = 0.002 mol L−1
Q(MgCO3) = [Mg2+][CO32−] = 0.001 × 0.002 = 2 × 10−6 < Ksp(MgCO3)
Hence MgCO3 will not precipitate.
HCl(aq) + KOH(aq) → KCl(aq) + H2O(l)
V(KOH) = 7.15 × 10−3 = 0.00715 L
n(KOH) = c × V = 0.10 M × 0.00715 L = 0.000715 mol
n(HCl)excess in 20 mL aliquot = 0.000715 mol (1:1 ratio)
n(HCl)excess in 100 mL = 0.000715 mol × 5 = 0.003575 mol
n(HCl)initial = c × V = 0.550 M × 0.1 L = 0.0550 mol
n(HCl)reacted = n(HCl)initial − n(HCl)excess = 0.0550 − 0.003575 = 0.051425 mol
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + H2O(l) + CO2(g)
n(CaCO3) = ½ × n(HCl)reacted = ½ × 0.051425 = 0.0257125 mol
m(CaCO3) = n × MM = 0.0257125 mol × (100.09 g mol−1) = 2.573564125 g
%m/m(CaCO3) = (2.573564125 / 3.0) × 100 = 85.8% (round to your desired s.f.)
Therefore, Brand X was used as 85.5% is the closest value.
Graph indicates acid (HA) is monoprotic, hence reacts with NaOH in a 1:1.
n(NaOH) = c × V = 0.10 × 0.024 = 0.0024 mol = n(HA)
[HA]initial = n/V = 0.0024 / 0.010 = 0.24 mol L−1
[H3O+]equilibrium = 10−2.5 mol L−1
| Concentration (mol L−1) | HA(aq) | A−(aq) | H3O+(aq) |
| Initial | 0.24 | 0 | 0 |
| Change | −10−2.5 | +10−2.5 | +10−2.5 |
| Equilibrium | 0.24 − 10−2.5 | 10−2.5 | 10−2.5 |
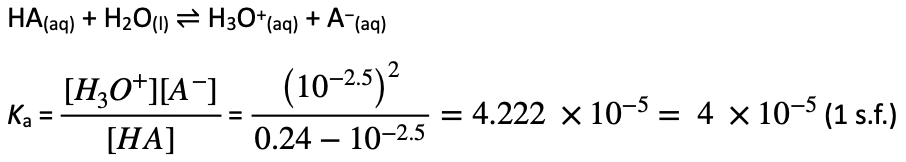
Alternative method using half-equivalence point:
At the half-equivalence point, the concentration of HA and A− will be equal, hence Ka = [H3O+] and pKa = pH.
Half-equivalence point occurs at 0.012 L. At this point, the pH = 4.4, so pKa = 4.4.
Ka = 10−pKa = 10−4.4 = 4 × 10−5 (1 s.f.)
As excess NaOH is added to the flask, the 0.10 mol L−1 solution is diluted by the salt solution produced from the neutralisation. This means the concentration of NaOH will continually be less than 0.10 mol L−1.
Since pH = 14 − pOH, if [OH−] = [NaOH] < 0.10 mol L−1, then pH < 14 − (−log100.010) < 13.
The energy profile diagram provided indicates the forward reaction is exothermic as the enthalpy of products is less than the enthalpy of reactants.
When a system is cooled from 80 °C to 0 °C, it disturbs the equilibrium. According to Le Chatelier’s principle, when a system is cooled it will minimise the disturbance by favouring the exothermic reaction to replenish the heat lost. Hence, the exothermic forward reaction is favoured, producing more pink [Co(H2O)6]2+ and thus a pink solution.
The colour change can also be explained using collision theory. When a system is cooled, both the rate of the forward and reverse reactions decrease, as there is less kinetic energy in the system, reducing the proportion of reactants that can collide with enough energy to exceed the activation energy. However, the decrease in the rate of the endothermic reverse reaction will be greater as the endothermic reaction has the greater activation energy (Ea2) in comparison to the forward exothermic reaction (Ea1). As a result, it experiences a greater proportional decrease in the number of collisions that exceed the activation energy, hence rate(reverse) < rate(forward). This means the rate at which pink [Co(H2O)6]2+ is produced is greater than the rate of production of blue [CoCl4]2−, producing a pink solution at equilibrium.
IR spectrum:
The IR spectrum would have a broad signal between 2500–3000 cm−1 due to the O–H bond of the carboxyl group and a sharp signal between 1680–1750 cm−1 due to the C=O bond of the carboxyl group.
13C NMR spectrum:
The 13C NMR spectrum would show 3 signals as there are three unique carbon environments in the molecule, as labelled A, B and C in the diagram below.
“A” carbon is directly bonded to electronegative oxygen, hence will be most deshielded and produce the most downfield signal between 160–185 ppm.
“B” carbon will produce a signal between 20–50 ppm. It is more upfield than “A” carbon as it is adjacent to a carbonyl group and not directly bonded to an electronegative atom.
“C” carbon will produce a signal between 5–40 ppm. It is the most upfield as it is the furthest from the electronegative oxygen.
1H NMR spectrum:
The 1H NMR spectrum would show 3 signals as there are three unique hydrogen environments in the molecule, as labelled a, b and c in the diagram below.
“a” proton is directly bonded to electronegative oxygen, so would produce the most downfield signal. The proton is acidic so is readily exchangeable and the adjacent carbon is not bonded to any protons, hence the signal will be a broad singlet.
“b” protons are bonded to a carbon that is adjacent to a C=O and has a neighbouring carbon with 3 protons that are in a different environment to itself. Thus “b” protons will be a quartet between 2.1–4.5 ppm.
“c” protons are bonded to a carbon that is adjacent to a CH2 group. Thus “c” protons would be a triplet between 0.7–2.1 ppm.
The signal integrals for Ha:Hb:Hc would be 1:2:3, which correspond to the number of protons in each environment.
Mass spectrum:
The mass spectrum would have a parent peak at m/z 74, which corresponds to the intact molecular ion that has a molar mass of 74 g mol−1.
There will be fragment peaks with m/z values less than 74. Possible fragment peaks could include:
The formula C5H10 for Compound A matches the general formula of an alkene CnH2n. It is also mentioned that compound A undergoes a reaction with H+/H2O which are the reaction conditions for hydration (it requires a dilute acid catalyst like sulfuric acid and H2O), which is an addition reaction that alkenes can undergo.
When an alkene undergoes a hydration, it produces an alcohol. It is noted that two products B and C are produced, indicating the alkene is asymmetrical. Since Compound B does not oxidise in the presence of acidified dichromate, it is a tertiary alcohol. This indicates one of the carbon atoms in the C=C in Compound A must be bonded to an alkyl group.
Compound C does react with acidified dichromate, indicating it is a primary or secondary alcohol which could produce either a carboxylic acid product or ketone when oxidised respectively. Since Compound D, the product of oxidation from Compound C, does not react with Na2CO3, Compound D is not a carboxylic acid and is a ketone indicating Compound C must be a secondary alcohol. This indicates the carbons in the C=C in Compound A are not terminal carbons.
Hence compound A is 2-methylbut-2-ene.
(Compound B is 2-methylbutan-2-ol, compound C is 3-methylbutan-2-ol and compound D is 3-methylbutan-2-one)
Written by Matrix Science Team
The Matrix Science Team are teachers and tutors with a passion for Science, across Biology, Chemistry and Physics, and a dedication to seeing Matrix Students achieving their academic goals.© Matrix Education and www.matrix.edu.au, 2025. Unauthorised use and/or duplication of this material without express and written permission from this site’s author and/or owner is strictly prohibited. Excerpts and links may be used, provided that full and clear credit is given to Matrix Education and www.matrix.edu.au with appropriate and specific direction to the original content.