Welcome to Matrix Education
To ensure we are showing you the most relevant content, please select your location below.
Select a year to see courses
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Select a year to see available courses
Science guides to help you get ahead
Science guides to help you get ahead
Ready to test yourself for the VCE Chemistry exams? This guide gives you 20 exam-style questions, along with key topics and expert tips to help you prepare.
Join 75,893 students who already have a head start.
"*" indicates required fields
You might also like
Related courses

Join 8000+ students each term who already have a head start on their school academic journey.
VCE Chemistry is a subject that builds on everything you’ve learnt, from atomic structure in Unit 1 to complex organic reactions in Unit 4. But to truly excel in your VCE Chemistry exams, you need to know how to apply it all.
To help you revise, we’ve created a free Matrix worksheet packed with practice questions covering the trickiest areas of the course and tips on how to solve them.
But before you dive into the worksheet, use this quick study guide to get clear on what to focus on.
For the full, detailed guide on the VCE Chemistry exam, read VCE Chemistry Study Guide: How to Ace Your Exam.
This VCE Chemistry study guide for Units 3 & 4 covers:
Some Chemistry topics are consistently tricky. They’re not impossible, but they require you to combine different concepts. You also need to think logically when under pressure.
Multi-step synthesis questions can feel like solving a chemical puzzle. You need to know your reactions and be able to link them in the right order.
Students often mix up the roles of electrodes and the direction of electron flow. In these questions, you’ll also need to interpret observations in practical scenarios.
Analytical Chemistry questions are like detective work. You’ll receive spectral data and need to deduce the structure of an unknown compound.
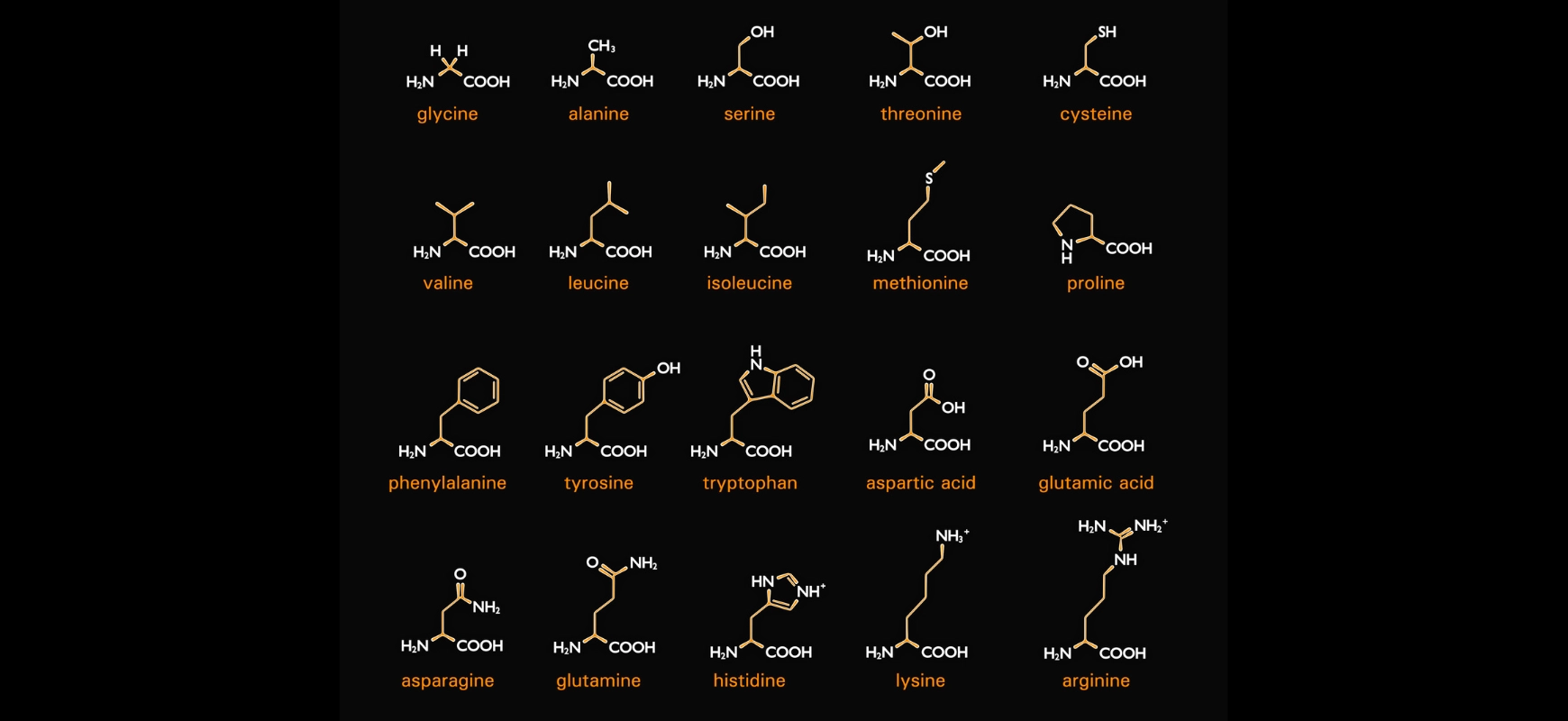
These questions often combine structure, function, and reaction knowledge (especially with proteins, fats, and carbohydrates).
Even strong students lose marks here by misreading the question or forgetting to find the limiting reagent.
Level up in VCE Chemistry with expert teachers
Master every topic with structured lessons and proven resources. Join 8000+ students learning one term ahead.
To maximise your revision for VCE Chemistry exams, focus your time and effort on these key areas:
This underpins many SACs and exam questions. Key topics in quantitative Chemistry and stoichiometry include:
Always convert to moles first!
Why it matters: If you’re fluent with these basics, you’ll be able to solve any reaction-based calculation confidently. Errors here usually come from skipping steps or unit mistakes, so always double-check your answers!
You need to master:
Why it matters: Redox appears in both theory and practical questions quite often. It’s usually worth a lot of marks and weight in the VCE Chemistry exams. Make sure you’re confident using the electrochemical series.

Know how to identify and connect reactions involving alkanes, alkenes, alcohols, carboxylic acids, amines, and more. You’ll be expected to map multi-step pathways and identify intermediates.
Why it matters: This section brings together IUPAC naming, functional group knowledge, and synthetic logic.
You’ll be tested on IR, H-NMR, MS, and chromatography. Make sure you understand what each technique reveals and how to interpret data.
Why it matters: These are real-life applications of Chemistry and frequently appear as extended-response questions. This means you’ll need to know how to answer using multiple forms of evidence.
Includes amino acids, proteins, carbohydrates, and fats. Questions often involve drawing structures, identifying reactions, or linking properties to biological roles.
Why it matters: Understanding biomolecular Chemistry helps connect abstract concepts to real-world systems, which is a major theme in Unit 4.
Here are two sample questions pulled directly from the Matrix worksheet. They target common exam traps and include quick tips to guide your approach. Let’s see how you’d handle them under exam-style conditions.
Write a full reaction pathway converting propene to propan-1-amine. Include all reagents, conditions, and intermediate products. (3 marks)
Tip: You’ll need to think about addition of HBr, substitution with ammonia, and then reduction. Don’t skip the naming!
A galvanic cell is constructed using Fe²⁺/Fe and Cu²⁺/Cu half-cells. Write the half-equations, label the anode and cathode, and calculate the cell potential. (3 marks)
Tip: Use the standard reduction potential table. Remember oxidation happens at the anode and electrons flow to the cathode.
FREE worksheet with exam-style questions to prepare you for the VCE Chemistry exam. Fill out your details below to get this resource emailed to you. "*" indicates required fields
Test your VCE Chemistry Unit 4 knowledge!

Test your VCE Chemistry Unit 4 knowledge!
Here’s how to make every study session count:
Matrix Chemistry students revise using this exact approach, in class and at home. Through structured lessons, practice quizzes, and expert feedback to close knowledge gaps and build exam readiness.
Get ahead in Science with expert teachers
Expert teachers and comprehensive resources to help you achieve more in Years 7-12 Science.
Written by Matrix Science Team
The Matrix Science Team are teachers and tutors with a passion for Science, across Biology, Chemistry and Physics, and a dedication to seeing Matrix Students achieving their academic goals.© Matrix Education and www.matrix.edu.au, 2025. Unauthorised use and/or duplication of this material without express and written permission from this site’s author and/or owner is strictly prohibited. Excerpts and links may be used, provided that full and clear credit is given to Matrix Education and www.matrix.edu.au with appropriate and specific direction to the original content.